Opisthorchis felineus and Metorchis bilis Metacercariae in Cyprinid Fish Leuciscus idus in Nura-Sarysu River, Kazakhstan
Article information
Abstract
Aim of the present study was to provide presence of opisthorchiid metacercariae in cyprinid fish Leuciscus idus in Nura-Sarysu river, Kazakhstan. Infection rate of the ides by the metacercariae was 42%. The metacercariae, similar morphologically to those of the liver flukes, were found: elliptical in shape, 0.19–0.25×0.15–0.22 mm, oral and ventral suckers nearly equal size, and excretory bladder O-shape with black content, occupying posterior part of the body. The metacercariae were divided into 2 groups with differences in size and thickness of cyst wall. Adult flukes were recovered from the Syrian hamsters infected with the opisthorch metacercariae and identified with morphological characters to Opisthorchis felineus and Metorchis bilis. DNA sequences of ITS1, ITS2, and cox1 supported the taxonomic assignment.
INTRODUCTION
Liver flukes of the family Opisthorchiidae (Looss, 1899) are considered as causative agents of serious disease worldwide, with the clinical pathology associated principally with chronic bile ducts infection with high risk of cholangiocarcinoma emergence [1–5]. All species within the family are obligate endoparasites with a complex 3-host life cycle including Bithyniidae snails and Cyprinidae fishes as the first and the second intermediate hosts and various piscivorous mammals and birds as definitive hosts [6,7].
Opisthorchiid flukes Opisthorchis felineus, O. viverrini, Clonorchis sinensis, and Metorchis bilis infect in the liver of mammals including humans [5,8,2,9–12]. Among these, O. felineus has been documented in humans and/or animals in 13 countries of the European Union [13]. This liver fluke also causes public health problem in the Russian Federation, Ukraine, Belarus and Kazakhstan [14].
Prevalence of opisthorchiasis reached a peak, 2,521 cases (17 cases per 100,000 population) in 2002, and gradually decreased to 1,225 cases (7.4 cases per 100,000) in 2011 in Kazakhstan [15]. Infection cases with M. bilis have rarely been reported in Kazakhstan. In Russia O. felineus and M. bilis were the main agents of liver fluke infection of humans living in the Ob-Irtysh river basin. This river basin was the largest endemic area of opisthorchiasis O. felineus with 50–63% of clinically diagnosed patients having mixed infection [11,16,17].
There are methods of diagnosing the disease but, have some drawbacks. Faecal examination for detection of parasite eggs and serological studies by ELISA are very laborious and fail to accurately distinguish the species of pathogen [18–22]. The development of highly sensitive and specific molecular genetics methods for the detection of trematodes of the family Opisthorchiidae and the precise identification of their species affiliation are an important urgent problem with a great epidemiological and medical significance [23–26]. Aim of this study was to identify the metacercariae of the family Opisthorchiidae in the Nura and Sarysu river basin Kazakhstan, using morphological and molecular genetic methods.
MATERIALS AND METHOD
Sample Collection
The 112 ides – Leuciscus idus (Linnaeus) (Cyprinidae) were caught in Sholak Lake, located in Nura-Sarysu basin (Korgalzhyn district, Akmola region, Kazakhstan). The fishes were individually tested for the presence of the trematode metacercariae by the muscle compression method. Infected fish specimens were used to isolate metacercariae by the artificial digestion method [8].
Experimental animals
Ten Syrian golden hamsters (Mesocricetus auratus) at the age of 8–10 weeks were kept under good hygienic conditions at the vivarium of Saken Seifullin Kazakh Agrotechnical University (KATU), and their use and care were approved by the Animal Ethics Committee of Veterinary Medicine Faculty of KATU. All of the procedures were in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). The hamsters were infected each orally with 50 metacercariae freshly isolated. The infection in the hamsters was verified by the coproovoscopy [27]. On the 40th day post infection (p.i.) hamsters were anesthetized with CO2 and euthanized, and adult trematodes were recovered from their livers for morphological and moleculargenetic studies.
Molecular analyses
For DNA extraction, 1/3 of the adult worm was homogenized separately in centrifuge tube and the method described previously [28]. DNA was dissolved in ddH2O and stored at −70°C. The polymerase chain reaction (PCR) was applied to identify opisthorchiid species using 3 primer pairs targeting nuclear ribosomal internal transcribed spacer 1 (ITS1: forward 5′-GTCGTAACAAGGTTTCCGTA-3′ and reverse 5′-ACACGAGCCGAGTGATCC-3′), ITS2, (forward 5′-GAACATCGACATCTTGAACG-3′, reverse 5′-GGAACGACCTGAACACCA-3′) and mitochondrial DNA, cytochrome oxidase subunit 1 (cox1: forward 5′-GGGTTTGGAATGATTAGTC-3′ and reverse 5′-CACAGAGGCAGAAAGAACT-3′) [29–32]. As second step, amplicons of ITS1 and 2 others were sequenced and analysed for species identification. As controls, primer pairs of O. felineus and/or M. bilis were designed to amplify a part of the mitochondrial cox1 gene [33]. In addition, a primer pair was used as a control to detect of O. viverrini metacercariae from fish [34]. The primers were synthesized by a company (Integrated DNA technologies, Houston, Texas, USA).
Amplification of the targeted genes was carried out in a final reaction volume of 50 μl containing 1×Phusion HF buffer, 2.5 mM MgCl2, 1 U Phusion DNA polymerase and 200 μM dNTPs (New England BioLabs Inc., Ipswich, Massachusetts, USA), 25 pmol of each primer and 20 ng DNA extracted from a single liver fluke specimen as template.
PCR was performed as follows: denaturation at 95°C for 50 sec, primer annealing at 65°C for 50 sec, and extension at 68°C for 7 min with a final extension for 5 min at 72°C. The resulting restriction fragments were separated by electrophoresis on an ethidium bromide containing 1.5% agarose gel using 1X TAE buffer solution. A PCR-amplified target gene fragment was purified using E.Z.N.A. Gel Extraction kit (Omega Biotek, Norcross, Georgia, USA), following the manufacturer’s protocols. The purified DNA was sequenced at the MD Anderson Cancer Centre, according to the Guidelines for DNA Submission. The resulting nucleotide sequences were visually checked by the Bio Capt program version 11.0. The nucleotide sequences of the studied species were compared with other sequences in the NCBI GeneBank database using BLAST. The phylogenetic analysis was carried out with MEGA 6 software.
The nucleotide sequences of the studied species were deposited in NCBI GenBank data base (O. felineus isolate 0827-AKKz01: ITS1, MG952283, ITS2, MG952281; M. bilis isolate 0829-AKKz02: ITS1, MG952284, ITS2, MG952282).
RESULTS
Morphology of the metacercariae
The opisthorchiid metacercariae isolated from all they were identified by gross morphology: elliptical cyst shape, 0.19–0.25×0.15–0.22 mm in size, equal size of oral and ventral suckers, and O-shaped excretory bladder filled with black excretory granules and occupying large part of posterior body [8,11,35]. Two species metacercariae were recognized morphologically very close, almost undistinguishable (Fig. 1). Their differences could be noted only in the size of cyst and thickness of cyst wall. Their variability of the features was overlapped, thus reduced differentiation capacity.
Recovery of adult flukes
Infection of the hamster was monitored daily after 30 days p.i. by coproovoscopy. The eggs of the opisthorchiid flukes were found in fecal pellets of the hamster on the 30–32 days p.i. Morphological features of the eggs were well compatible to opisthorchiid eggs. The eggs were light yellow or grayish, elongated to oval, slightly asymmetric, having a lid on one end and a small bump on the other pole, measured 23.0–35.0×10.0–20.0 μm. Morphology of the eggs revealed high similarity in shape and size, thus it was hard to assign them to either opisthorchiid species.
At post-mortem examination on the 40th day p.i, adult liver flukes were recovered from the bile ducts and/or gall bladder of all infected hamsters, with a recovery rate 35±5.4% (17.5±2.7 flukes per hamster). The eggs collected after incubation of the recovered flukes were found as a mix of opisthorchiid eggs, very similar in shape and size. Based on the morphological features, the recovered adult flukes were identified to O. felineus or M. bilis (Fig. 2).
The O. felineus adult was lancet-shaped, narrow anteriorly to the end and rounded at the tail. Body size was 5–7 mm long and 1–1.5 mm wide [35]. The oral and ventral suckers were almost equal in size. The oral sucker was round, diameter 0.26–0.28 mm, subterminal, slightly flattened near the pharynx. The ventral sucker, round, diameter 0.24–0.25 mm, sited approximately anterior 1/5 of the body. The pharynx, 0.14–0.16 mm×0.15–0.17 mm, located just posterior to the oral sucker. The esophagus, 0.15–0.16 mm long, passed down and bifurcated into 2 intestinal caeca laid in the lateral fields and stretched to posterior end of the body. The ovary is smoothedged, weakly lobed and localized in front of the testis in middle of the body. The uterus wound up from the ovary to front edge of the ventral sucker. The genital pores opened at anterior side of the ventral sucker. The vitelline glands as 7–8 bunches, aligned long both marginal fields lateral to the intestinal caeca in middle of the body. The seminal receptacle was behind the ovary. The testes were 4–5 lobed and located diagonally one after another. The described morphological features of the fluke shown on Fig. 2A corresponded well to those of O. felineus.
The M. bilis adults had leaflike form, body size was 2–4 mm long and 1–1.5 mm wide (Fig. 2B). The oral and ventral suckers were almost equal in size. The oral sucker was round, diameter 0.22–0.28 mm, subterminal. The ventral sucker, round, diameter 0.10–0.30 mm, shifted forward from middle of the body. The pharynx was oval, diameter 0.12–0.13 mm. The esophagus was very short, practically absent. The ovary was smooth-edged, rounded, localized in front of the testis and slightly lateral from central axis of the body. The uterus wound up from the ovary and overlaps the ventral sucker, reaching approximately the same level as the vitelline glands. The genital pores opened anterior to the ventral sucker. The vitelline glands distributed from front level of the anterior testis or ovary and reach half level between the 2 suckers. The seminal receptacle was behind the ovary, equal to or larger than the ovary. The anterior and posterior testes were smooth-edged and located obliquely in hind quarter of the body. The seminal vesicle was quite crooked [11,36]. Significant overlapping in morphology between M. bilis and other species in genus Metorchis was reported [36,37].
The trematodes recovered were identified by morphological features each to O. felineus and M. bilis.
Molecular identification
Five adult flukes each of O. felineus (1st group) and M. bilis (2nd group) were used for molecular phylogenetic analysis. The PCR products were separated on 1.5% agarose gel by electrophoresis (Fig. 3). Primer pairs ITS1 and ITS2 allowed to amplify 664 bp and 525 bp fragments from O. felineus and M. bilis, respectively. The PCR for cox1 produced 260 bp amplicon of O. felineus, but not of M. bilis (Fig. 3A, B).

Electrophoretic analysis of PCR products obtained with DNA of the adult trematodes. (A) Opisthorchis felineus. (B) Metorchis bilis: Lanes 2–7, primer pair of ITS1, ITS2, cox1, Of, Mb and Ov, respectively. Lane 1, DNA ladder (bp); lane 8, negative control.
The OF primer pair amplified a specific 200 bp fragment from the O. felineus DNA but, did not from the M. bilis DNA. The MB primer pair neither produce amplicon from O. felineus or from M. bilis (Fig. 3B).
The PCR-amplified DNA fragments of the 1st and 2nd groups were purified from agarose gel and sequenced. As a result, we successfully obtained ITS1, ITS2, cox1 sequences of O. felineus and M. bilis in Nura-Sarysu basin.
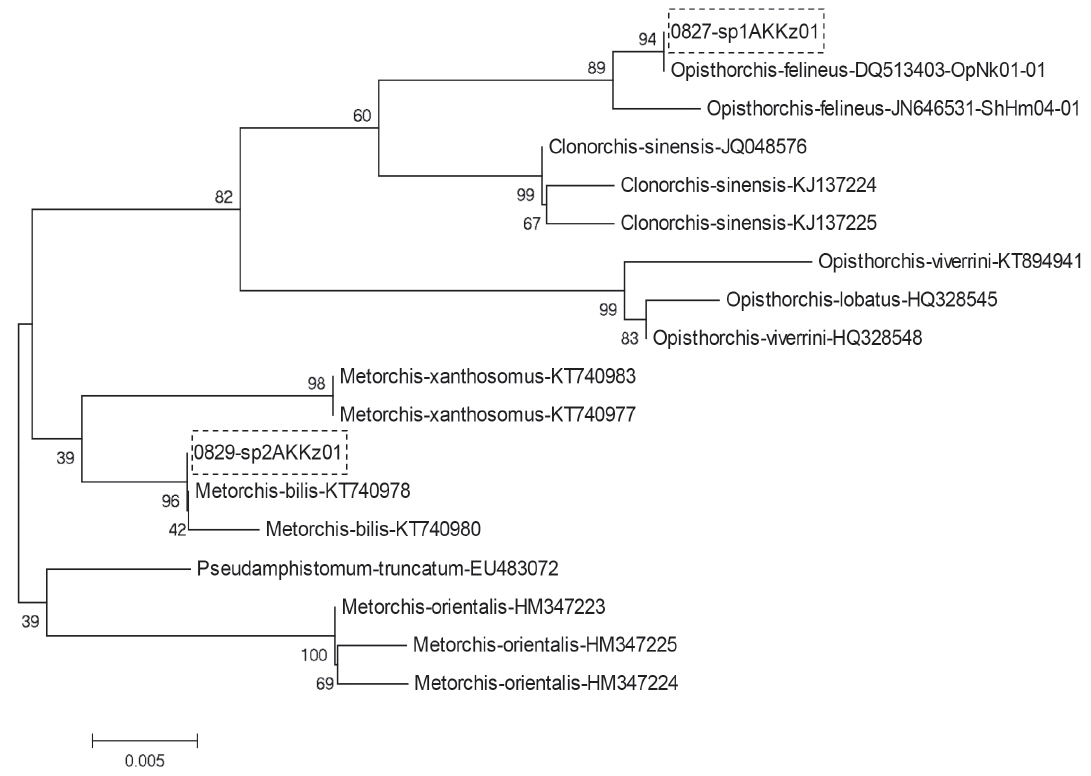
The BLAST analyses approved that the 1st group was highly similar to O. felineus and the 2nd group was highly similar to M. bilis [37].
These similarity wasillustrated with results of phylogenetic analysis using MEGA 6 software. The ITS1 phylogenetic tree constricted by ML algorithms certainly assigned the 1st group to O. felineus and the 2nd group to M. bilis (Fig. 4). The same result was obtained with ITS2 sequences of the 1st and 2nd groups (not shown).
DISCUSSION
In the Ob-Irtysh rivers basin – the world’s largest endemic focus of O. felineus-associated opisthorchiasis, the majority of patients with clinical symptoms of opisthorchiasis were infected simultaneously with O. felineus and M. bilis [11,24,25]. Therefore, it is very important to have reliable methods for differentiation the metacercariae species in intermediate hosts for accurate epidemiologic prognosis and effective treatment and prevention measures development. The microscopic method of differentiating metacercariae is very laborious, time-consuming, and requires qualified specialists. The reliable methods that make possible to distinguish the larvae of trematodes from each other can be only based on molecular genetics approach [26].
Among the population living in the Nura-Sarysu basin, that is one of the 8 basins of Kazakhstan, opisthorchiasis is widespread, but mixed opisthorchiidiases have not been registered yet. If consider other intermediate hosts, there are only minor references to a mixed infection (O. felineus and M. bilis) in the study area. For example, necropsy investigations of village dogs in an endemic region revealed 37 of 51 (72%) village dogs infected with either Opisthorchis felineus or Metorchis bilis. Two dogs were infected with single specimens of Metorchis bilis. However, the authors did not provide accurate (genetic) data on the presence of 2 species of parasite and emphasized that it was not possible to confirm whether the human opisthorchiidiasis was caused by O. felineus or M. bilis [15].
The present study provided the results of morphological and molecular identification of the trematodes of the family Opisthorchiidae circulating in the lakes of the Nura-Sarysu basin as metacercariae (encysted larvae) in ides. The choice of the fish species is explained by the data on previous studies reporting the Leuciscus idus living in the lakes of Nura-Sarysu basin to be the most infected with the metacercariae of the opisthorchiids [39]. Extensity of infection of the Leuciscus idus by Opisthorchiidae in the Sholak lakes of the Nura-Sarysu basin was 42%.
The comparative description of morphology for O. felineus and M. bilis metacercariae was provided by S. Beer [8]: in encysted state they both have round or oval shape with thin transparent walls with size of O. felineus one being 0.17÷0.34×0.21÷0.43 mm versa M. bilis one being 0.13÷0.16×0.19÷0.23. Thus the 2 species encysted metacercariae are recognized as morphologically very close, almost undistinguishable [8]. Despite this, we managed to distinguish 2 different types, the differences of which were in size of cyst and thickness of cyst. But the differentiation of the 2 species flukes is more effective at their adult stage.
The hamsters experimentally infected with the larvae of opisthorchiids showed high susceptibility to infection. External and internal structure of the trematodes indicated that they belonged to 2 species: O. felineus and M. bilis. A molecular study was carried out to confirm the assignment.
In our study the previously described primers ITS1, ITS2 and cox1 were used mainly for opisthorchiid DNA amplification [29–31]. The amplified DNA fragments of adult trematodes were then sequenced for species identification. It should be noted that we failed to amplify the DNA fragments of M. bilis not only by using cox1 primers, but also with the help of MB primers which design was based on the sequence of M. bilis cox1 gene sequence [33]. The inability to amplify M. bilis DNA fragments using the primers mentioned requires further detailed study.
Thus, the ide living in the lakes of the Nura-Sarysu basin are additionally proved by molecular genetic technique to be invaded by metacercariae of 2 species of Opisthorchiidae trematodes infective for mammals including humans: O. felineus and M. bilis. These data indicate the high rate of mixed infection among the population of the Akmola region, which must be taken into account in the diagnosis and treatment of people from trematodes, as well as in the development of disease prevention measures.
ACKNOWLEDGMENTS
The authors are grateful to Dos D. Sarbassov (MD Anderson Cancer Center, Texas University, Houston, USA) who helped with specific primers and sequencing of PCR-amplified DNA fragments and Lyudmila A. Lider (S. Seifullin Kazakh Agro-Technical University, Astana, Kazakhstan) for their technical support in fish testing for the presence of metacercariae. This investigation was financially supported by the Ministry of Education and Science of the Republic of Kazakhstan within the frame-work of the projects No. 0115 RK00487 for 2015–2017, AP05131132 for 2018-2020. The molecular genetic work was partially supported by the project #0324-2018-0016 of the FRC ICG SB RAS and the Russian Foundation for Basic Research (grant numbers 15-04-08893).
Notes
CONFLICT OF INTEREST
The authors declare that there are no competing interests related to the publication of this paper.