Acanthamoeba in Southeast Asia – Overview and Challenges
Article information
Abstract
Acanthamoeba, one of free-living amoebae (FLA), remains a high risk of direct contact with this protozoan parasite which is ubiquitous in nature and man-made environment. This pathogenic FLA can cause sight-threatening amoebic keratitis (AK) and fatal granulomatous amoebic encephalitis (GAE) though these cases may not commonly be reported in our clinical settings. Acanthamoeba has been detected from different environmental sources namely; soil, water, hot-spring, swimming pool, air-conditioner, or contact lens storage cases. The identification of Acanthamoeba is based on morphological appearance and molecular techniques using PCR and DNA sequencing for clinico-epidemiological purposes. Recent treatments have long been ineffective against Acanthamoeba cyst, novel anti-Acanthamoeba agents have therefore been extensively investigated. There are efforts to utilize synthetic chemicals, lead compounds from medicinal plant extracts, and animal products to combat Acanthamoeba infection. Applied nanotechnology, an advanced technology, has shown to enhance the anti-Acanthamoeba activity in the encapsulated nanoparticles leading to new therapeutic options. This review attempts to provide an overview of the available data and studies on the occurrence of pathogenic Acanthamoeba among the Association of Southeast Asian Nations (ASEAN) members with the aim of identifying some potential contributing factors such as distribution, demographic profile of the patients, possible source of the parasite, mode of transmission and treatment. Further, this review attempts to provide future direction for prevention and control of the Acanthamoeba infection.
INTRODUCTION
Acanthamoeba spp. is one of pathogenic free-living amobae (FLA) along with Naegleria fowleri, Balamuthia mandrillaris, and Sappinia sp. which are potential to cause rare infection in central nervous system. These protozoan parasites are mostly found in natural soil and water bodies and immunocompromised patients as the main target [1]. Recently, Acanthamoeba spp. are recognized as increasing threat against contact lens wearers and healthy individuals also take some risks on amoebic keratitis (AK) [2]. Understanding on Acanthamoeba infections is therefore crucial but still limited in ASEAN countries even though studies on anti-Acanthamoeba agent do exist. Herein, an overview of Acanthamoeba was put in a nutshell as well as challenges on recent issues to encounter against this amoeba in our regional ASEAN countries including Brunei Darussalam, Cambodia, Indonesia, Lao People’s Democratic Republic (PDR), Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam.
ORIGIN OF ACANTHAMOEBA
Acanthamoeba spp. is a centrosome-bearing, single-celled, flattened naked amoeba in Order Acanthopodida, Class Centramoebia, Phylum Discosea, Amoebozoa clade in Amorphea domain of Eukaryotic organisms [3]. Term “Acanth” in Greek means spike representing prominent sub-pseudopodia while “amoeba” means alteration like their appearance. The bacteria-phagocytosing protozoa is one of clinical FLA ubiquitous in nature soil and water bodies as well as man-made environment as a secondary decomposer. Ubiquity is implied by presence of antibodies in healthy individuals [4]. Acanthamoeba sp. was first recognized as contaminant of Cryptococcus pararoseus culture by Castellani in 1930 and named as Hartmannella castellanii and then a year later, Acanthamoeba spp. because of its double-walled cyst with irregular ectocyst appearance which is different from round and smooth cyst wall of Hartmannella spp. [5].
BRIEF BIOLOGY OF ACANTHAMOEBA
Acanthamoeba spp. appears in 2 forms of life cycle: trophozoite (25–40 μm) and cyst (13–20 μm). Trophozoite is an infective stage with amoeboid locomotion whilst cyst is a dormant stage against harsh environment such as temperature and pH imbalance, malnutrition, or presence of anti-Acanthamoeba agents [6]. One third of strength of cyst wall might come from polymer of glycosidic linkages between saccharides while another 2/3 are protein and other components, respectively [7]. Furthermore, the protist acts as potential reservoir or vector of human-pathogenic bacteria, fungi, or viruses while endosymbiont and Acanthamoeba-resistant organisms also are identified [8–10]. Recently, more than 25 species were recorded in NCBI taxonomy database and 20 genotypes were published which T4 is a major genotype associated with human infections [9,11]. For cultivation, xenic culture is obtained by using non-nutrient (Page’s amoeba saline) or PYG (peptone 0.05%, yeast extract 0.05%, glucose 0.1%) agar coated with living or killed bacteria (e.g., Escherichia coli) at 25–28°C in the dark for 2–3 days for trophozoite proliferation and 1–2 weeks for encystment while PYG (peptone 2%, yeast extract 0.5%, glucose 0.5%) agar was used for axenic culture [12]. Culture in PYG medium at 4°C would be convenient method for long-term preservation at least 1–4 years [13].
EPIDEMIOLOGY OF ACANTHAMOEBA IN ASEANS
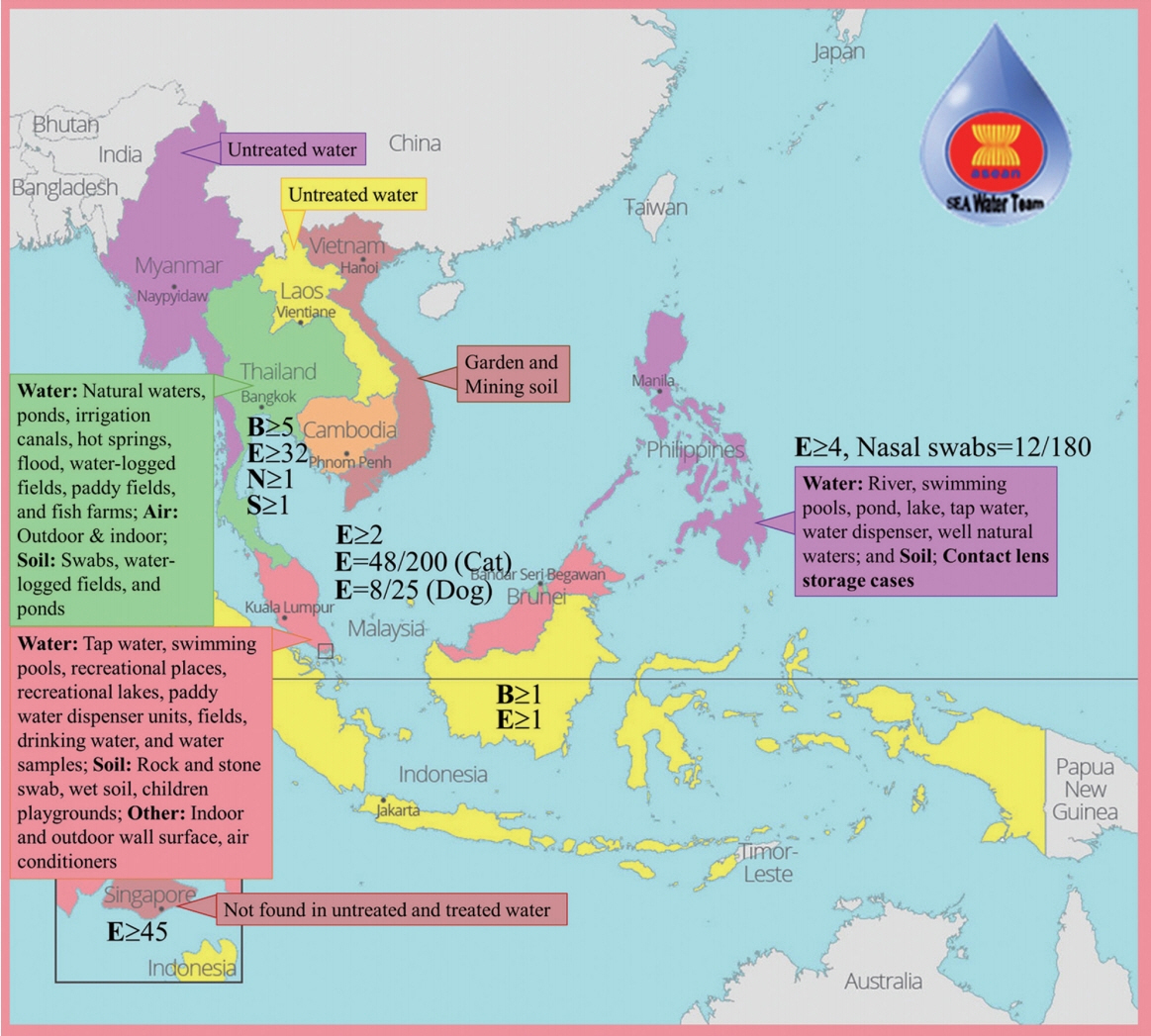
FLA, especially Acanthamoeba spp., occur worldwide and have a variety of habitats. Many studies have recorded the wide distribution in soil and water, with differing range of thermal tolerance (Table 1). They have been isolated in untreated natural freshwaters, like lakes, ponds, hot springs and waterfalls [14–17]; and brackish, seawaters, and ocean sediments [18]. They were also isolated from treated waters like domestic water systems, swimming pools, hydrotherapy pools, remedial spas, tap water and drinking water [14,16,19,20]. Unconventional water sources like sewage and aquaria were not spared with the presence of amoebas [18].
Aside from water, Acanthamoeba spp. were also present in different types of soils such as agricultural, garden and mining [21–23]. Acanthamoeba genotypes of infected cats and dogs were matched with dry soil and dust. [24]. Acanthamoeba-infected individuals can also be a source of the isolates of organism through sinuses, brain and corneal and skin specimens [22,25–27] and even in necrotic tissues [18].
The presence of Acanthamoeba spp. has impacted for the last decades because of the increasing cases of a rare condition AK, a severe infection of the eye cornea associated with intense pain. This has been observed in contact lens wearer population [28]. It is believed that the cause of infection is due to the exposure of the eye to the Acanthamoeba-contaminated contact lens solutions. Acanthamoeba isolated from contact lens storage cases were confirmed [29]. Further, the usual spread of the contaminant is due to poor hygiene and maintenance of the lens; and exposure to contaminated water (swimming pool or other recreational waters) while wearing contact lenses.
However, the disease has also been reported in non-contact lens wearers [18,26,27]. This further affirms the possible contamination through direct contact to contaminated water and soil. The wide dispersal of Acanthamoeba onto the environment is due to the wind dispersal of its resistant form, the cysts. Likely that indoor ventilation system, blowing fan, air diffuser and other furniture contaminated with Acanthamoeba can be a cause of spreading indoor [30]. Thus, individuals who are not contact lens wearers but have been constantly exposed to dust particles and soil are also at high risk of infection [25]. It is also important to note that exposure to Acanthamoeba can be as simple as accidental splash of contaminated water to the face or bruised skin [14], making a fast and easy transmission.
Ironically, with the many studies proving the presence of Acanthamoeba in different environmental media (soil, water and air), the dearth of information in Southeast Asian (ASEAN) countries is quite a concern, considering that the varying climatic conditions of the region is a favorable habitat for this organism which has an unusual geographic distribution [31].
The ASEAN countries’ tropical condition, favorite tourist destinations during summer, consists of beaches, falls, and lakes are among the popular areas where more people involve with these outdoor activities. The congestion can increase risk of contamination with Acanthamoeba especially when the environment is dry during summer and dust particles can be easily spread. Likewise, resorts with swimming pools are occupied the entire summer with local and foreign tourists. Since resorts gain profit only during this time of the year, owners tend to maximize the use of the swimming pools which may compromise the proper cleanup of the swimming facility. This poses the risk to the swimmers, adding to the fact that Acanthamoeba can also be resistant to disinfectants [26,32].
The detection of Acanthamoeba in soil, water and air in other countries in ASEAN (Fig. 1), confirms that a continual contamination of the environment persists, and this poses a risk to people dependent on the soil and water for domestic activities, agricultural and farming occupation, and even for recreation. The lack of information in some countries (Cambodia and Brunei) does not mean the absence of Acanthamoeba-contaminated environment. Albeit, this may result to the inability of one country to control the spread of possible diseases associated with Acanthamoeba considering that this amoeba may also harbor pathogenic bacteria or fungi.
CLINICAL SIGNIFICANCE AND DIAGNOSIS
Potential pathogenicity of Acanthamoeba was first observed in monkey kidney cell in vitro as well as intracerebral/intraspinal inoculation in monkeys and intravenous/intranasal inoculation in mice [33,34]. First patient was recognized as GAE in 1972 and a year later, AK [35,36]. Acanthamoeba spp. are therefore considered as rare potential pathogen causing cutaneous lesions, sinusitis, AK, GAE, and disseminated form in human and prefer individuals with underlying diseases or immunocompromised host but AK was frequently reported in immunocompetent patients especially, contact lens wearers [37].
For AK, poor sanitation of contact lens wearer is a potential risk and corneal trauma seem required before trophozoite infection as well as eye secretion after contact lens wore might be preferred by Acanthamoeba [38,39]. Onset of AK is days to weeks with symptoms of tormenting eye pain, redness, photophobia, stromal infiltration leading to sight-threatening condition which are similar and misdiagnosed to Herpes simplex, bacterial or fungal keratitis [39,40]. AK is confirmed by presence of trophozoite with large nucleolus and contractile vacuoles as well as pseudopodia and transparent protrusions of Acanthopodia from corneal scrapings or biopsies under direct microscopy with several stains. Encystment on non-nutrient agar (NNA) and nucleic acid amplification testing are further investigated for species identification and genotyping, respectively. Taxonomic identification mainly investigated by cyst morphology under microscope [41] and a hypervariable sequence part of 18S small subunit rDNA gene called ASA.S1 by Acanthamoeba-specific primers: JDP1 and JDP2 (amplicon size 467 bps for Neff strain of A. castellanii accession number M13435.1) [42]. Extended or almost complete of 18S rDNA amplicon size provide better solution for genotyping [11,42]. Pathogen broad-spectrum and most effective anti-Acanthamoeba agents against two forms, 0.02% polyhexamethylene biguanide (PHMB) or chlorhexidine, still need antibacterial, antifungal, or aromatic diamidines combination because of resistance of cyst form and PHMB is toxic to human corneal cells [40].
For GAE, a very rare condition, is opportunistic and fatal infection with onset of weeks to months mostly in immunocompromised patients, especially HIV/AIDS patients through skin breaks, respiratory tract, and olfactory epithelium. GAE patient will encounter with neurological signs such as confusion, headache, and stiff neck as well as psychological change, e.g. irritability generally like other brain infections due to effect of edema, necrosis, and hemorrhages in infected part of brain [43]. To confirm GAE, microscopy and culture from CSF remain gold standard methods used after neuroimaging detection of brain lesions while indirect immunofluorescence on tissue and multiplex real-time PCR assay are available [44]. Late/missed diagnosis, blood-brain barrier crossing of antimicrobial, drug side effects, drug combination are still an issue on GAE treatment and only few patients were cured [45,46].
There are many reports on Acanthamoeba infection in ASEAN countries (Table 2). Most infections are AK with contact lens while cases of GAE is rare. Notably, Acanthamoeba can be involved with gastric ulcer and sinusitis and found from nasal swab from healthy individuals and corneal swab from infected animal (Table 2). Undeniably, exposure to soil and contaminated water are potential risk but underlying disease might be another one factor for the infections. Misdiagnosis and delay in diagnosis are common among patients leading to permanent vision blurriness because of injured cornea or deeper layers for AK and death for GAE. These problems are still insolvable till date. Rapid and accurate prognosis is therefore an urgent need for Acanthamoeba infection.
CURRENT CHALLENGES AND FUTURE PERSPECTIVES
Contact with Acanthamoeba spp. is common. Immunocompromised patients should realize this risk and avoid exposure to, especially, natural soil and water bodies even though it is a rare disease but GAE is fatal and AK is vision-threatening [6]. Moreover, no specifically therapeutic course is available for Acanthamoeba spp. infections, in case of GAE. However, commercial drugs for AK are highly toxic due to prolonged treatment duration as well as diagnosis and combination of treatments depends on medical expertise of physician and availability of resources [6,47]. The statement diagnostic is challenge that a new molecular technology can be used in Acanthamoeba detection and monitoring system to understand these amoebic infections and diagnostic approaches. So far, the gold standard of Acanthamoeba laboratory testing has been cultured on NNA overlaid with E. coli and PYG medium for axenic culture. A modern technique has been applied as far as the laboratory diagnosis is concerned. This can provide a better routine diagnosis especially using molecular-based intervention such as PCR and MALDI-TOF/MS [5]. It is important because mistaken or late diagnosis has been usually reported due to poor prognosis leading to worsening clinical symptoms and subsequently under postmortem diagnosis [48,49]. Moreover, Acanthamoeba spp. are potential Trojan horse of human-pathogenic viruses, infectious bacteria, and fungi which might be one way of disease spread and gene transfer [10]. Early detection technique is needed as well as physician should be aware of Acanthamoeba infection through patient interview and history taking [50,51]. Unfortunately, most patients also come up with lesion in brain for GAE which most of the cases are too late to be cured whilst AK are mainly associated with contact lens wearer and immunocompetent patients are basically affected [52–54].
Hygiene and proper contact lens usage is a critical point of care which ophthalmologist should pass on knowledge of appropriate usage of contact lens [55]. Most disinfectant solutions for contact lens are ineffective against Acanthamoeba cyst which is rich with cellulose structure [56]. Effort on novel anti-Acanthamoeba agents therefore focus on cyst form or other potential target sites [7]. Biology of Acanthamoeba spp. should be studied to guide action of desired anti-Acanthamoeba agents which have been identified [57]. In ASEAN nations, Anti-Acanthamoeba activity has been investigated among human-made chemicals, plant extracts, microbial metabolites, anti-Acanthamoeba side effect of drugs, and animal products which nanoparticles are attractive antimicrobial agent delivery technology to enhance the activity of these anti-Acanthamoeba agents (Table 3). However, these anti-Acanthamoeba agents were tested only in vitro. Blood-brain barrier is another challenge for anti-Acanthamoeba agents to pass through for the treatment of GAE [58]. There is a long road lying ahead for in vivo experiment and clinical application in ASEAN nations. In fact, Southeast Asia (ASEAN) is a gigantic resource of medicinal plants and bioactive agents. Interestingly, the only PHARM database is available at the Faculty of Pharmacy, Mahidol University, Thailand in which more than 1,000 collections of Thai medicinal plants were recorded (http://www.medplant.mahidol.ac.th/pharm/search.asp: March 13, 2019). It is therefore noteworthy to strongly recommend for more research works that should be further explored on the plants-based medicinal therapy for severe or deadly infections with Acanthamoeba spp.
ACKOWLEDGMENTS
This work is under the project entitled of “Medicinal under-exploited Thai native plants against Acanthamoeba, Leishmania donovani, and Plasmodium falciparum – Toward South East Asia collaboration initiative (Grant No. 040226) supported by The Royal Patronage of Her Royal Highness Princess Maha Chakri Sirindhorn”. We are also grateful to the Project CICECO-Aveiro Institute of Materials, FCT Ref. UID/CTM/50011/2019.
Notes
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this study.