Detection of Human Anti-Trypanosoma cruzi Antibody with Recombinant Fragmented Ribosomal P Protein
Article information
Abstract
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi, and is endemic in many Latin American countries. Diagnosis is based on serologic testing and the WHO recommends two or more serological tests for confirmation. Acidic ribosomal P protein of T. cruzi showed strong reactivity against positive sera of patients, and we cloned the protein after fragmenting it to enhance its antigenicity and solubility. Twelve positive sera of Chagas disease patients were reacted with the fragmented ribosomal P protein using western blot. Detection rate and density for each fragment were determined. Fragments F1R1, F1R2, and F2R1 showed 100% rate of detection, and average density scoring of 2.00, 1.67, and 2.42 from a maximum of 3.0, respectively. Therefore, the F2R1 fragment of the ribosomal P protein of T. cruzi could be a promising antigen to use in the diagnosis of Chagas disease in endemic regions with high specificity and sensitivity.
Chagas disease, also known as American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi [1]. It is found in many Latin American countries and is responsible for significant health and economic burden in the area [2,3]. The disease spreads mainly through insect vectors, but can also be transmitted orally, congenitally, or through transfusion or organ transplantation in a lesser extent [1,4]. The highest incidences are located in resource-constrained areas and rural settings. The World Health Organization (WHO) estimates about 6 million to 7 million people worldwide, mostly in Latin American countries, are infected [5].
Chagas disease does not produce immediate or easily observed symptoms in individuals, but over time, if untreated, can lead to serious cardiac and digestive complications, resulting in loss of productivity and ultimately death. The initial acute phase lasts for roughly two months post infection and many individuals have either no symptoms or only mild symptoms during this time. In the acute phase parasites can be found circulating in the bloodstream and microscopic identification of parasites is recommended. In the subsequent chronic phase, the parasites sequester primarily in cardiac and digestive tissues, potentially causing gradual and severe damage to the organs. Diagnosis is based on serologic testing in asymptomatic individuals. Currently, the WHO recommends the use of 2 or more serological diagnostic tests for confirmation of the infection [6].
Patients’ sera were provided by Dr. Wagner Maricondi of the WAMA Diagnostica (Aldo Germano Clein, Sao Carlos, Brazil), which were collected according to the “Bioethics and Security” protocol, and only unnamed samples assigned with numbers under the regulation of the IRB Committee of WAMA Diagnostica (2 January 2014) were provided. Esmeraldo CL2 strain of T. cruzi was purchased from ATCC (ATCC® 50820, Manassas, Virginia, USA) and maintained in ATCC 1029 LIT media at 25°C in 25-T flask with 12–14 days passage. Western blot with patients’ sera to the whole extract of T. cruzi revealed that a 37 kDa protein was blotted strongly by the entire sera (data not shown). This 37 kDa protein is known as an acidic ribosomal P protein [7,8] and we fragmented the amino acid sequence prior to cloning to enhance its antigenicity and solubility, as designed in Fig. 1A. DNAs were amplified with multiple sets of primers as F1: 5′-CG GGA TCC GTG CAT GAC GTT CGT CGC GA-3′, F2: 5′-CCG GGA TCC GTG AGT GAC AAG AAG GTA CTG AGC-3′, R1: 5′-CCG CTC GAG TTA GAA CAG CGC CCC CAT-3′, R2: 5′-CCG CTC GAG AAA GTC GTC GTC ATC ATC CTC CTC T-3′ and R3: 5′-CCG CTC GAG GCT CAG TAC CTT CTT GTC ACT CAC-3′ to insert into pGEX4T-1 plasmid, and then expressed as GST fusion proteins detected with HRP-conjugated anti-human IgG antibody (Sigma Aldrich, St. Louis, Missouri, USA) as shown in Fig. 1B. Twelve positive sera of Chagas disease patients were reacted with the fragmented ribosomal P protein (Table 1). Rate was determined as the detection rate for each fragment against positive patients’ sera. Density of the bands for each fragment against sera were measured as +, ++, and +++ arbitrarily, and scored into 1, 2, and 3. The averages were calculated and used as the final density. Fragments F1R1, F1R2, and F2R1 showed 100% rate of detection, and average density scoring of 2.00, 1.67, and 2.42 from a maximum of 3.0, respectively.
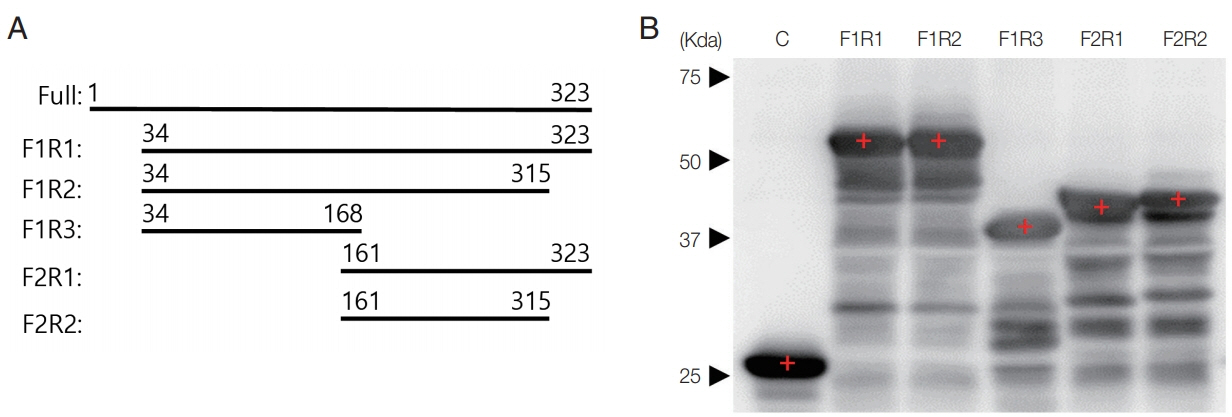
Production of recombinant P protein fragments. (A) Design of the fragmented acidic ribosomal P protein of T. cruzi. (B) Fragments detected with anti-GST antibody by western blot.
Ribosomal P proteins form a complex of proteins in the large subunit of the ribosome that is called the stalk [9]. These proteins act as a molecular switch in the ribosome related to the binding and release of elongation factors and GTP hydrolysis [10].
The C-terminal half of the ribosomal P protein of T. cruzi produce the autoantibodies responsible for Chagas disease. So, this high reactivity of the fragment F2R1 could be used for detecting positive Chagas disease patients. It might be a useful diagnostic antigen in immunoaffinity methods such as western blot, ELISA, and even rapid diagnostic test. Therefore, it would serve as a method of choice for point-of-care diagnosis and large-scale surveys in regard to T. cruzi infection among people under clinical or field conditions in endemic areas worldwide.
Notes
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this study.
