Soboliphyme baturini (Nematoda: Soboliphymatidae) Recovered from Stomach of Asian Badger, Meles leucurus, in Geochang-gun, Gyeongsangnam-do, Korea
Article information
Abstract
We are going to describe the female soboliphymid nematodes, which were recovered from the stomach of a Asian badger, Meles leucurus (Mammalia: Mustelidae), in Geochang-gun, Gyeongsangnam-do, Korea. In February 1998, we found 2 peculiar nematodes with a cup-like organ in the anterior end from the stomach of badger. Recovered worms were fixed with 10% formalin, cleared in glycerin-alcohol solution and observed under a light microscope with a micrometer. They were 34.46 (33.43–35.50) mm long by 2.13 mm at maximum width. Cephalic sucker cup-like, 3.34 (3.13–3.55) mm wide, 2.40 (2.25–2.55) mm long, with the oral aperture and meridionally striated on the buccal capsule. Oral aperture 2.38 mm in diameter. Circumoral membrane 0.41 (0.38–0.45) mm wide. Esophagus muscular, 4.81 (4.50–5.00) mm long by 0.80 (0.78–0.83) mm at maximum width. Vulva situated at 3.13 mm ventro-anterior level from the esophago-intestinal junction. Vagina anteriad, 3.38 mm long, making a canal from the uterus to the vulva opening. Uterus single, large. Tail 0.35 (0.33–0.38) mm long. Intrauterine eggs long elliptical, 0.058–0.065 (0.062) mm long and 0.030–0.033 (0.031) mm wide. Based on the some morphological characters and host-specificity, our specimens are nearly identical with S. baturini. Therefore, the present report describes S. baturini for the first time in Korea.
Nematode members in the genus Soboliphyme (Nematoda: Soboliphymatidae) are the gastro-intestinal parasites of carnivores and insectivores. About 9 species, i.e., Soboliphyme abei, S. ataahai, S. baturini, S. caucasica, S. huridiniformis, S. jamesoni, S. occidentalis, S. soricis, and S. urotrichi, have been reported in the literatures [1–7]. It has been known that soboliphymid nematodes have 2 hosts in their life cycle. Larvae develop in oligochaetes, earthworm, and adults dwell in the gastro-intestinal tract of insectivorous and carnivorous mammals by the eating of intermediate and/or paratenic hosts with larvae [2,3]. However, there are no reports on the soboliphymid nematodes in Korean fauna. We describe with 2 female soboliphymids recovered from the stomach of a Asian badger, Meles leucurus (Mammalia: Mustelidae), in Geochang-gun, Gyeongsangnam-do, Korea.
In February 1998, we obtained a whole set of visceral organs and some muscles of a badger from a wholesale house of animals located in Geochang-gun, Gyeongsangnam-do, the Republic of Korea (Korea) to examine the infection of Trichinella larvae. We also examined helminthic infections from the badger and recovered 2 peculiar nematodes with a cup-like organ in the anterior end from the stomach. Recovered worms were fixed with 10% formalin, cleared in glycerin-alcohol solution and observed under a light microscope with a micrometer.
They were female worms with a cup-like structure in the anterior and a pointed tail in the posterior ends. Their morphological characters were as follow (Fig. 1). Body 34.46 (33.43–35.50) mm long and 2.13 mm at maximum width. Cephalic sucker cup-like, 3.34 (3.13–3.55) mm wide, 2.40 (2.25–2.55) mm long, with the oral aperture and meridionally striated on the buccal capsule. Oral aperture (opening) 2.375 mm in diameter. Circumoral membrane 0.413 (0.38–0.45) mm wide. Esophagus muscular, 4.81 (4.50–5.00) mm long and 0.80 (0.78–0.83) mm at maximum width. Vulva situated at 3.125 mm ventro-anterior level from the esophago-intestinal junction. Vagina anteriad, 3.38 mm long, making a canal from the uterus to the vulva opening. Uterus single, large. Tail 0.35 (0.33–0.38) mm long. Intrauterine eggs long elliptical, 0.058–0.065 (0.062) mm long and 0.030–0.033 (0.031) mm wide (Table 1).
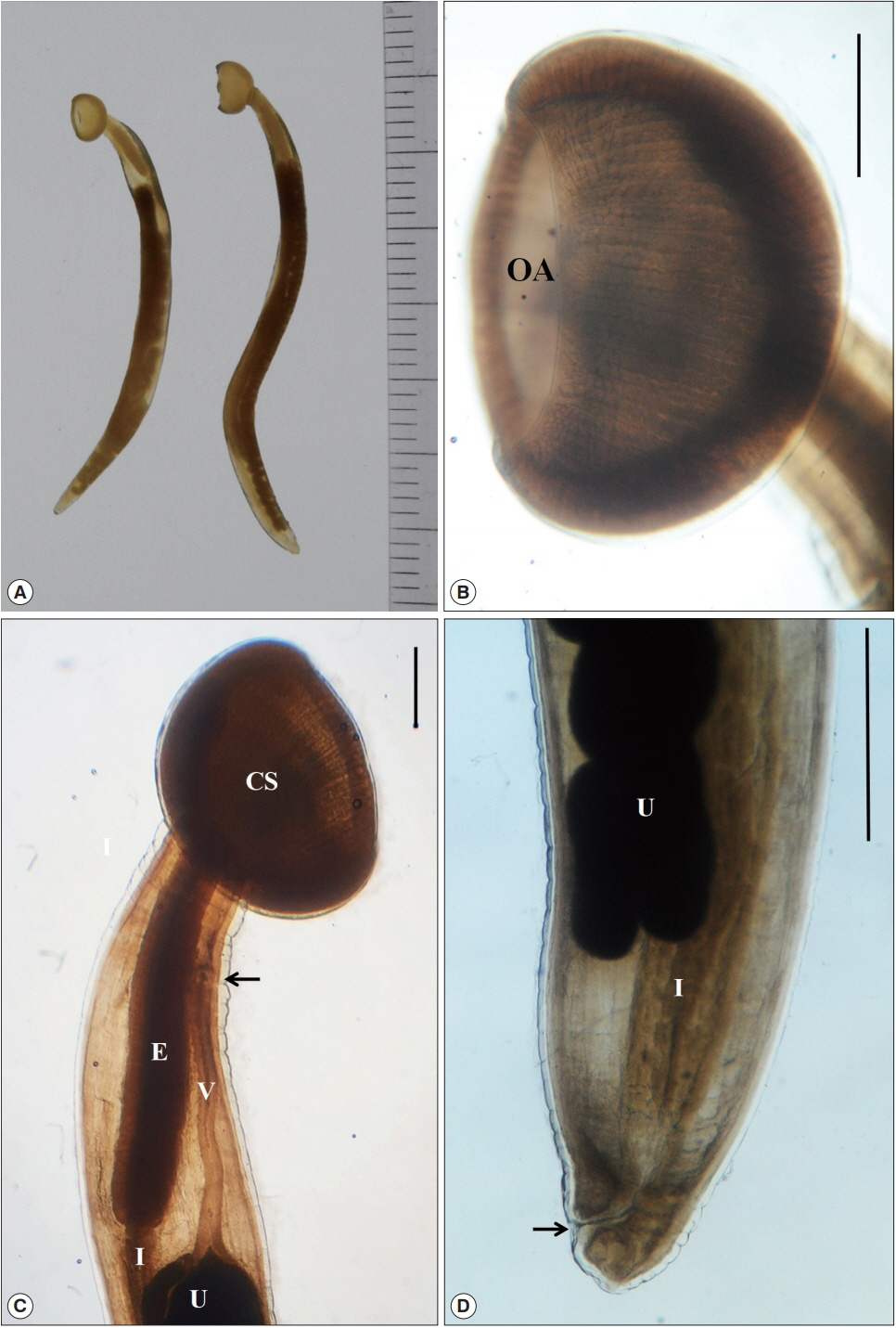
(A) Two female worms of Soboliphyme baturini recovered in the stomach of badger, Meles leucurus, from Geochang-gun, Gyeongsangnam-do, Korea. (B) Magnified view of cephalic sucker with the oral aperture (OA) and meridionally striated on the buccal capsule. (C) Anterior portion with a characteristic cephalic sucker (CS), muscular esophagus (E), intestine (I), uterus (U), long vagina (V) and vulva opening (arrow). (D) Posterior portion with the uterus (U), intestine (I) and anus (arrow). Scale bar=1 mm.
Among 9 soboliphymid nematodes reported in the literatures, 8 species, i.e., S. abei, S. ataahai, S. caucasica, S. huridiniformis, S. jamesoni, S. occidentalis, S. soricis and S. urotrichi, are detected in insectivores, shrews and moles. Only 1 species, S. baturini, has been found in carnivorous mammals in adult stage like in this study [1–7]. Over 32 species of mammals have been listed as the hosts of this nematode species [8]. Adult worms of S. baturini are most commonly detected in sable and marten (Martes spp.), and less frequently found in ermine and weasel (Mustela spp.), wolverine (Gulo gulo), and mink (Neovison vison) [8]. Larval and/or juvenile worms are found in shrew (Sorex spp.), canids and felids, which acted as the paratenic and incidental hosts in the life cycle. In the aspect of host-specificity, our specimen is closely related with S. baturini. Moreover, the European badger, Meles meles, has been listed as the definitive host of S. baturini [8–10].
Soboliphymids residing in insectivores are known to be host-specific and restrictive in distribution. Cross infections between soboliphymids of shrews and moles do not occur. Four Soboliphyme spp., S. abei, S. ataahai, S. jamesoni and S. soricis, have been detected from shrews and 4 species, S. caucasica, S. hirudiniformis, S. occidentalis and S. urotrichi, have been found from moles. On the other hand, 4 species, i.e., S. caucasica, S. hirudiniformis, S. occidentalis and S. soricis, are distributed in Europe only, 3 species, S. abei, S. ataahai, and S. urotrichi, appear to be restricted to Asia, and only one species, S. jamesoni, occurs in North America [3–5,11–14]. Only one species that parasitizes in carnivores, S. baturini, is broadly distributed in Eurasia and North America [3,15].
Six of the 9 soboliphymid nematodes previously reported, i.e., S. ataahai, S. baturini, S. huridiniformis, S. occidentalis, S. soricis and S. urotrichi, have the notched cephalic sucker. Among these, only 2 species, S. baturini and S. huridiniformis, have the vulva opening at the ventro-anterior from the esophago-intestinal junction like in our specimens (Table 1). However, S. huridiniformis is found only in the insectivores, moles. The 2 specimens in this study are considerably larger than those of previous descriptions for S. baturini [16,17], but similar with those of S. sahalinense Shimakura and Odajima, 1934 [18], which has been considered to be a synonym of S. baturini [2,3]. Some limited points, i.e., lack of male worms and insufficient number of female samples, exist in this study, but our specimens are closely identical with some morphologies of S. baturini.
Based on the some morphological characteristics and host-specificity, the soboliphymid nematodes recovered from the stomach of badger were identified as S. baturini in this study. Accodingly, we report for the first time that the Asian badger, M. leucurus, serves as the definitive host for S. baturini, and this species of nematode is distributed in Korean peninsula. Further studies on the soboliphymid nematodes should be performed in insectivores as well as in carnivores to expand the nematode fauna in Korea.
Notes
CONFLICT OF INTEREST
We declare that we have no conflict of interest related to this work.