Abstract
The seasonal abundance of hard ticks that transmit severe fever with thrombocytopenia syndrome virus was monitored with a collection trap method every April to November during 2015–2018 and with a flagging method every July and August during 2015–2018 in Ganghwa-do (island) of Incheon Metropolitan City, Republic of Korea. This monitoring was performed in a copse, a short grass field, coniferous forest and broad-leaved forest. A total of 17,457 ticks (8,277 larvae, 4,137 nymphs, 3,389 females, and 1,654 males) of the ixodid ticks comprising 3 species (Haemaphysalis longicornis, H. flava, and Ixodes nipponensis) were collected with collection traps. Of the identified ticks, H. longicornis was the most frequently collected ticks (except larval ticks) (94.26%, 8,653/9,180 ticks (nymphs and adults)), followed by H. flava (5.71%, 524/9,180) and Ix. nipponensis (less than 0.04%, 3/9,180). The ticks collected with collecting traps were pooled and assayed for the presence of SFTS virus with negative results. In addition, for monitoring the prevalence of hard ticks, a total of 7,461 ticks (5,529 larvae, 1,272 nymphs, 469 females, and 191 males) of the ixodid ticks comprising 3 species (H. longicornis, H. flava, and Ix. nipponensis) were collected with flagging method. H. longicornis was the highest collected ticks (except larval ticks) (99.53%, 1,908/1,917 ticks (nymphs and adults)), followed by H. flava (1.15%, 22/1,917).
-
Key words: Hard tick, severe fever with thrombocytopenia syndrome (SFTS), SFTS virus, Ganghwa-do, climate change
INTRODUCTION
Ticks are obligate blood-sucking ectoparasites of many hosts, including mammals, birds, reptiles, and even amphibians, and the second most common arthropod vector, which not only is direct damaged by biting but also transmit many pathogens, including bacteria, parasites, or virus [
1,
2]. They have been recognized by their possibility to transmit various tick-borne pathogens to their host. Ticks and their transmitting pathogens are among significant public health burdens worldwide and their ranges have expanded, contracted, and shifted. Life on earth largely depends on the dynamics of our climate system, especially earth’s surface climate. With the impact of climate change on public health becoming widely recognized, infectious pathogens have gained increased focus [
3,
4]. Actually, ticks are sensitive to global changes related to climate, vegetation, and landscape. Recent changes in socioeconomic and environmental situations, as well as the expanding geographic distribution of ticks mainly due to climate changes, have contributed to worldwide burden of tick-borne disease (TBD), along with growing numbers of potential tick-borne pathogens (TBPs) [
5,
6]. Among parasites, ticks are particularly considered from the perspective of climate change due to dependence on both biotic and abiotic conditions for their survival. Climate conditions, such as temperature, humidity and rainfall have been reported as predominant factors influencing the life cycle, activity and fecundity of tick populations [
7]. The incidence and geographic distribution of vector-borne diseases are anticipated to change as a result [
8]. Ticks are connected with their hosts for the time of the blood meal, during which period they undergo specific and intense interactions at the host interface. Therefore, local existence of ticks relies on the presence and abundance of proper hosts [
9]. Except eggs, ticks have 3 life stages, such as larva, nymph, and adult (male and female), all of which require a blood meal for further development. In general, ticks are vectors of a wide range of human and livestock disease agents. Thus, the factors for their distribution, such as climate conditions, host movements, and landscape modifications, are pivotal for predicting and elucidating disease occurrence and emergence [
10].
Severe fever with thrombocytopenia syndrome (SFTS) is a newly emerging viral hemorrhagic fever that was first discovered in China in 2009, where the infection manifested with major clinical symptoms of severe fever and thrombocytopenia [
11]. The incubation period is generally 6–14 days, with an average of 9 days. Ixodid tick species as well as
Haemaphysalis longicornis ticks that are extremely widespread in the Republic of Korea (Korea) are implicated as vectors of pathogenic SFTS bunyavirus [
12]. SFTS virus is a tick-borne virus, Family Bunyaviridae, Genus Phlebovirus, is the causative agent of SFTS. Notably, this virus cases were identified in Korea in 2012. The first case was retrospectively identified on a sample collected in August 2012 from a female patient with history of insect bites while working on a crop farm and who died of multiple organ failure [
13]. The overall incidence of this disease in Korea was 0.11 cases per 10
5 person-years, which was lower than that in China (0.12–0.73 cases per 10
5 person-years) [
14,
15]. To date, the disease is one of group 4 infectious diseases in Korea according to the Korean Act on the Prevention and Control of Infectious Diseases. The annual number of cases was more than 800 since 2013 (36 in 2013, 55 in 2014, 79 in 2015, 165 in 2016, 272 in 2017, and 259 in 2018) raising significant public health burdens [
16]. The case fatality rates reported were 47.2% in 2013 by Korea Centers for Disease Control and Prevention (KCDC) and 32.6% during 2013–2015 by Choi et al. [
15,
17]. SFTS signs and symptoms were high fever (≥38°C), vomiting, diarrhea, and/or fatigue and showed laboratory parameters consistent with thrombocytopenia and/or leukocytopenia [
18]. However, Bae et al. argued that Korean SFTS patients presents with significantly more diarrhea and confusion compared to a group of Chinese patients [
19]. Although person-to-person transmission has been reported [
20,
21], the virus is mainly transmitted to humans by means of bite of SFTS virus-infected ticks.
This effect of climate change and unsustainable human activities intimidates health security worldwide, finally directly jeopardize public health. This issue of climate change is to contribute to scarcity not only through increased temperatures and prolonged drought times, but also through the degradation of water resources carrying pathogens and other contaminants, which pose significant health risks. Thus, there is clearly a strong need for establishing management strategy and constant monitoring the resources. For a fact, for establishing strategies to control and prevent TBD, it is necessary to comprehend how many different tick species are ranged in different regions, which species are reservoirs of pathogens, and elucidate which factors could facilitate the occurrence of tick vectors [
22,
23]. In the present study, we conducted large-scale 4-year surveillance of ticks in Ganghwa-do (Ganghwa) of Incheon Metropolitan City (Incheon), Korea. We report here on the species composition, species diversity, abundance, and distribution of ticks and their pathogens to monitor and reduce the potential for autochthonous transmission of TBPs, especially SFTS virus, due to the effect of climate change in Korea. The results could provide the basis for future epidemiological studies and risk assessment of TBPs as an effect of climate change in Korea.
MATERIALS AND METHODS
Collection sites and sample collection
Ganghwa lies in the Yellow Sea of Korea’s Western coast and the estuary of Han river. This island is the 4th largest island in Korea. The average temperature for the year is 16.2°C and precipitation is at 1,346 mm per year. The climate classification of Ganghwa is Dwa [
24].
Collection of the ticks for occurrence surveillance was performed in a copse, a short grass field, a coniferous forest and a broad-leaved forest with a trap method every April to November during 2015–2018 with 3 collection traps (total 12 traps) at each site in Ganghwa. In addition, for 2 months (July and August) for 4 years (2015–2018), ticks were collected from 4 habitats, copse geographic index [GI: 37.7344966/126.397436], short grass field [GI: 37.7333344/126.395645], coniferous forest [GI: 37.733885/126.396880], and broad-leaved forest [GI: 37.732803/126.398165]) by flagging (20 times of each) across the vegetation surface. Ticks were sampled at each site on the same day in each year. Ticks collected by flagging method with 1 m
2 flag (1×1 m) were used for the surveillance of distribution in 2 sites and the SFTS virus detection. In these areas, no specific permission was required for collecting ticks, and this study did not involve endangered species. The collected ticks were identified to the species level and stage based on their morphological features [
25]. However, in case of larvae collected, they were not used as data for species distribution due to difficulties of their identification.
The collected ticks were pooled (5 for adults and 30 for larva and nymph) by species, collection site, and time of survey and quickly transferred to microcentrifuge tubes on ice. To improve the extraction and to achieve high efficiency and reduced variability in the total RNA yields, the extraction was carried out in bead beaters with 2.8 mm stainless steel beads (Precellys
TM Hard tissue homogenizing CK28-R, Bertin Technologies SAS, France) with Trizol
TM reagent (Invitrogen, Carlsbad, California, USA). Tick pools were homogenized in 500 μl Trizol
TM reagent. After the tick homogenates were centrifuged at 14,000 rpm for 5 min, the supernatant was collected and RNA extracted according to the recommended Protocol for Direct Zol
TM RNA extraction kit (Zymo Research, Irvine, California, USA). The extracted total RNA was resuspended in RNase-free water containing RNAsin
TM Plus RNase inhibitor (Promega, Medison, Wisconsin, USA) and then stored at −70°C until use. RT-qPCR assays were conducted using DiaStar
TM 2× OneStep RT-PCR pre-mix (SolGent Co. Daejeon, Korea) in accordance with the manufacturer’s protocol. The SFTS virus-specific primers used were as follows: MF3 (Sense) 5′-GATGAGATGGTCCATGCTGATTCT-3′ and MR2 (Anti-sense) 5′-CTCATGGGGTGGAATGTCCTCAC-3′ for the medium (M) segment of SFTS virus [
26,
27], and Actin L (Sense) 5′-AGATCATGTTCGAGACCTTC-3′ and Actin R (Anti-sense) 5′-TCAGGATCTTCATCAGGTAA-3′ for β-actin. RT-qPCR was performed using 15 μl 2× OneStep RT-PCR Pre-mix, each with 10 pmole primer, and 1 pg to 100 ng total RNA in a 30 μl total reaction volume. RT-qPCR conditions for each reaction were 30 min at 50°C, followed 15 min at 95°C, then 20 sec 95°C, 40 sec 58°C and 30 sec at 72°C for 35 cycles, and finally annealed for 5 min at 70°C. Samples were determined as SFTS virus positive by monitoring the 560 bp DNA fragment band on 1.5% agarose gel electrophoresis stained with 0.1 μl/ml of SYBR
TM Safe DNA gel stain (Invitrogen).
RESULTS
Collection of vector hard ticks using collection trap
A total of 17,457 ticks (8,277 larvae, 4,137 nymphs, 3,389 females, and 1,654 males) of the ixodid ticks comprising 3 species (
H. longicornis, H. flava, and
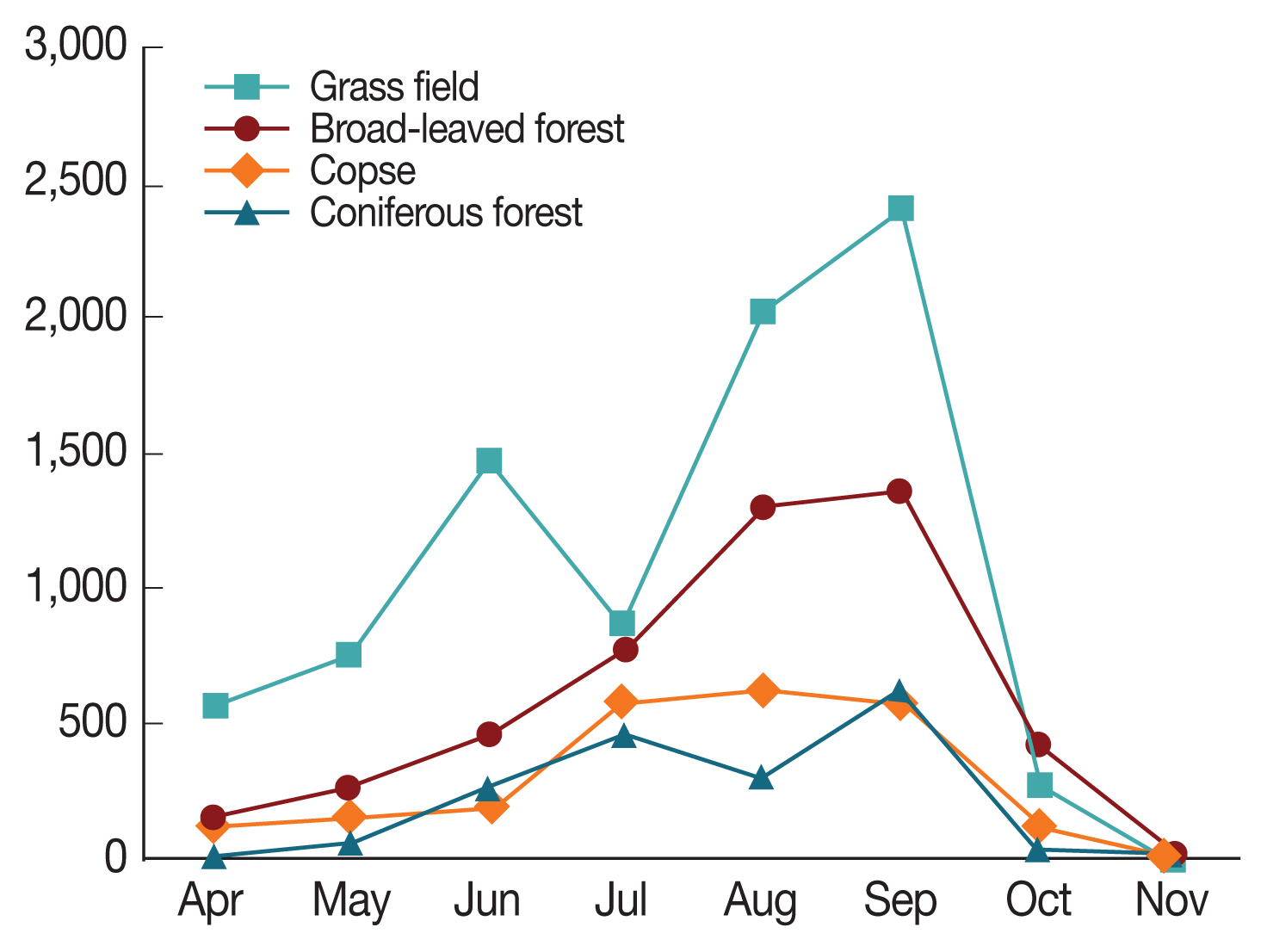
Ixodes nipponensis) were collected with collection traps and assayed for the presence of a novel SFTS virus causing an infectious disease, SFTS from April to November 2015–2018 at 4 locations (copse, short grass field, coniferous forest, and broad-leaved forest) in the Ganghwa area of Incheon, Korea. Overall, the most abundantly collected location was the short grass field (48.03% [8,385/17,457] with hard tick indexes [HTI, total number of hard ticks divided by total number of used traps] of 87.36), followed by the broad-leaved forest (28.03% [4,893/17,457], HTI of 49.67), the copse (13.89% [2,424/17,457], HTI of 25.25), and the coniferous forest (10.05% [1,755/17457], HTI of 18.28) (
Table 1;
Fig. 1). Given the similarity of the temperature and relative humidity in same island, differential host density is likely to be the major contributing factor to inter-site difference. Generally, taking the results with collection traps, it is shown that short grass field is more favorable than copse as a habitat for ticks. All of the 3 species collected in this study were found to bite humans, as reported in other studies [
28]. Of the identified ticks,
H. longicornis that are known to be widespread in Korea was the most frequently collected ticks (except larval ticks) (94.26%, 8,653/9,180 ticks [nymphs and adults]), followed by
H. flava (5.71%, 524/9,180) and
Ix. nipponensis (less than 0.04%, 3/9,180) (
Table 2). On possibility of these tick distribution between
H. longicornis and
H. flava is that
H. longicornis are ectoparasites of mammals, whereas
H. flava are those of birds and mammals [
29]. The population density of
Ix. nipponensis was too low to be considered vector for SFTS in our study sites. This prevalence shows a finding coincident with many studies in Korea [
12,
26,
27,
30]. The nymphal hard ticks were collected 4,137 individuals (23.70%), the females were 3,389 (19.41%), and the males were 1,654 (9.47%) and larvae were 8,277 (47.41%). The ratio of total numbers of each instar collected 8,277 larvae, 4,137 nymphs, and 5,043 adults) was broadly in no line with expectations. In general, peaks of female adults, male adult, nymph, and larva numbers were observed from July to August, from June to July, from May to June, and from August to September, respectively.
H. longicornis populations increased from April to July peaking in July (2,584) and
H. flava showed bimodal collection rates, with a high number collected during June (75), then the highest again during September (176) (
Table 2).
A total of 7,461 ticks (5,529 larvae, 1,272 nymphs, 469 females, and 191 males) of the ixodid ticks comprising 3 species (
H. longicornis,
H. flava, and
Ix. nipponensis) were collected with flagging method (
Table 3). Hard ticks were counted by dragging a white woolen blanket measuring 100×100 cm across the vegetation surface at July and August 2015–2018 at 4 locations (copse, short grass field, coniferous forest, and broad-leaved forest) in the Ganghwa area. Of the identified hard ticks,
H. longicornis was the highest collected ticks (except larval ticks) (99.53%, 1,908/1,917 ticks [nymphs and adults]), followed by
H. flava (1.15%, 22/1,917). The nymphal hard ticks were collected 1,272 individuals (17.05%, 1,272/7,461), the females were 469 (6.29%, 469/7,461), the males were 191 (2.56%, 191/7,461) and the larvae were 5,529 (74.11%, 5,529/7,461). The ratio of total numbers of each instar collected (5,529 larvae, 1,272 nymphs, and 660 adults) was broadly in line with expectations. However, the overall ratio of adult males to female was not ordinary. Males persist on vegetation for longer, looking for repeat mating. The most frequently collected location was the copse (46.50%, 3,469/7,461), followed by the broad-leaved forest (28.74%, 2,144/7,461), the short grass field (18.31%, 1,366/7,461) and the coniferous forest (6.46%, 482/7,461). Nymphs in short grass field persisted for rather locations (
Table 3).
The collected ticks were pooled (5 for adults and 30 for larva and nymph) by species, collection site, and time of survey for screening SFTS virus. The 560 bp identified DNA bands were used for the pathogen detection based on the sequence of partial M segment [
26,
27]. The collected ticks were divided into 1,057 pooled groups by sites, species, and stage in preparation and tested for SFTS viral RNA detection. None of the pooled of ticks were positive for SFTS virus by RT-qPCR assays.
DISCUSSION
To date, the climate of Korea has gradually changed into a subtropical region, and this might make Korean Peninsula environmentally suitable for the proliferation of vector ticks in the near future. Climate change is an unequivocal phenomenon and is expected to accelerate in the Korean Peninsula where the extent of climate change is largely being felt [
31]. In fact, ticks are particularly considered from mutual relation of climate change due to dependence on environmental conditions for their survival.
The ixodid ticks belong to the 4 species from 2 genera:
H. longicornis, H. flava, Ix. nipponensis, and
Ix. persulcatus [
30]. SFTS virus has previously been reported in ticks belonging into 3 genera and 4 species (
H. longicornis, H. flava, Amblyomma testudinarium, and
Ix. nipponensis) [
32]. Recently, SFTS virus was detected in all developmental stages (eggs, adults, nymphs, and larvae) of
H. longicornis ticks in China [
33].
H. longicornis is the most dominant species of hard ticks in Korea, and almost all tick-borne diseases in Korea are known to originate from
H. longicornis [
25,
32,
34,
35]. In addition, SFTS virus has also been isolated from
A. testudinarium and
Ix. nipponensis, which may serve as additional vectors in Korea [
26]. The seasonal distribution of the collected ticks in this study could be reflected their life cycle, the peak transmission in summertime may be attributable to the seasonal life cycle of
H. longicornis and the increased rates of outdoor activity during this season [
12]. We determined that
H. longicornis is widely distributed in Ganghwa area as the dominant tick species (94.26% with collection trap method and 99.53% with flagging method) and that it was not infected with SFTS virus in our studying locations. Although other tick species were also collected in our studying locations beyond
H. longicornis with both flagging and trap methods, there might be more tick species than those collected in this study in Ganghwa area [
36]. These results should be useful for creating awareness of STFS occurrences in this area and for the provision of public health information. In Korea, the prevalence of SFTS virus in ticks collected from vegetation was 0–0.46% [
12,
26,
37]. From the study of the prevalence of SFTS virus in 5 national parks of Korea, the infection rate of SFTS virus in adult and nymph ticks was 3.61% and that of larval ticks was 0.31% [
38]. The total tick numbers, the dominant stage of ticks, outdoor activities, whether change, and other factors could contribute to the SFTS occurrence. The different sampling methods yielded complementary information on the presence of hard ticks in different locations of the vegetation and at different month, reflecting the combination of vegetation structure and hard tick biology. The results of this study represent in a plain term how their usefulness in assessing hard tick hazards for humans may be diminished by missing ticks in other locations as its structure changes seasonally. Prevalence is one of the important tools in the study of the epidemiology of ticks and tick-borne parasites. And the role of ticks as vectors and infected portion are pivotal tools for an adequate tick control program, especially in terms of climate change. In our view, the relatively small-scale monitoring of hard ticks presented here for each location are comparable because hard ticks were collected by the same investigators over the same short period for 4 years. The finding in this monitoring is in rough terms consistent with many previous observations [
12,
13,
26,
27,
30]. It is also shown that seasonal patterns detected by the dissimilar methods were roughly similar.
In conclusion, SFTS is a prevalent endemic disease in Korea that has a high fatality ratio. Thus, it is necessary to carefully and continuously monitor the hard ticks as a strong transmitter of various tick-borne pathogens, especially SFTS virus in terms of climate change. It is reported that the annual mean temperature has been increasing at a rate of 0.52°C per decade. Although this study represents none of SFTS virus infection in ticks collected at Ganghwa, monitoring and studying the hard ticks at various vegetation is required for a better understanding and preparing of their potential impact on public health.
Notes
-
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this article.
ACKNOWLEDGMENTS
This study was supported by fund from the Korea Centers for Disease Control and Prevention (4851-304-320) in the Republic of Korea and by the Inha University Research Fund (INHA-6027).
Fig. 1Distribution of the collected tick densities of each collection location from April to November 2015–2018.

Table 1Collection rate and hard tick index for 8 months in Ganghwa during 2015–2018 using collection traps
Table 1
|
Collection sites |
Month |
No. of collection traps |
No. of collected hard ticks |
Hard tick index (HTI) |
|
Copse |
Apr. |
12 |
132 |
11.11 |
|
May |
12 |
160 |
13.33 |
|
Jun. |
12 |
201 |
16.75 |
|
Jul. |
12 |
580 |
48.33 |
|
Aug. |
12 |
631 |
52.58 |
|
Sep. |
12 |
590 |
49.17 |
|
Oct. |
12 |
128 |
10.67 |
|
Nov. |
12 |
2 |
0.17 |
|
Subtotal |
|
96 |
2,424 |
25.25 |
|
|
Short Grass Field |
Apr. |
12 |
567 |
47.25 |
|
May |
12 |
766 |
63.83 |
|
Jun. |
12 |
1,478 |
123.17 |
|
Jul. |
12 |
856 |
71.33 |
|
Aug. |
12 |
2,033 |
169.42 |
|
Sep. |
12 |
2,417 |
201.42 |
|
Oct. |
12 |
266 |
22.17 |
|
Nov. |
12 |
1 |
0.08 |
|
Subtotal |
|
96 |
8,385 |
87.36 |
|
|
Coniferous Forest |
Apr. |
12 |
7 |
0.58 |
|
May |
12 |
67 |
5.58 |
|
Jun. |
12 |
259 |
21.58 |
|
Jul. |
12 |
459 |
38.25 |
|
Aug. |
12 |
299 |
24.92 |
|
Sep. |
12 |
626 |
52.17 |
|
Oct. |
12 |
37 |
3.08 |
|
Nov. |
12 |
1 |
0.08 |
|
Subtotal |
|
96 |
1,755 |
18.28 |
|
|
Broad-leaved Forest |
Apr. |
12 |
152 |
12.67 |
|
May |
12 |
266 |
22.17 |
|
Jun. |
12 |
589 |
49.08 |
|
Jul. |
12 |
786 |
65.5 |
|
Aug. |
12 |
1,305 |
108.75 |
|
Sep. |
12 |
1,362 |
113.5 |
|
Oct. |
12 |
419 |
34.92 |
|
Nov. |
12 |
14 |
1.67 |
|
Subtotal |
|
96 |
4,893 |
49.67 |
|
|
Total |
|
384 |
17,457 |
45.14 |
Table 2Monthly distribution of hard ticks in Ganghwa, 2015–2018, using collection traps
Table 2
|
Species |
Stage |
Apr. |
May |
Jun. |
Jul. |
Aug. |
Sep. |
Oct. |
Nov. |
Total (%) |
|
Haemaphysalis longicornis
|
Female |
80 |
281 |
618 |
1,501 |
728 |
31 |
24 |
3 |
3,266 |
|
Male |
61 |
155 |
452 |
701 |
109 |
5 |
2 |
0 |
1,485 |
|
Nymph |
684 |
818 |
1,309 |
382 |
164 |
537 |
8 |
0 |
3,902 |
|
Subtotal |
|
825 |
1,254 |
2,379 |
2,584 |
1,001 |
573 |
34 |
3 |
8,653 (49.57) |
|
|
H. flava
|
Female |
6 |
7 |
6 |
5 |
10 |
40 |
44 |
4 |
122 |
|
Male |
4 |
5 |
10 |
1 |
19 |
69 |
55 |
6 |
169 |
|
Nymph |
17 |
45 |
59 |
6 |
8 |
67 |
31 |
0 |
233 |
|
Subtotal |
|
27 |
57 |
75 |
12 |
37 |
176 |
130 |
10 |
524 (3.00) |
|
|
Ixodes nipponensis
|
Female |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Male |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Nymph |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
|
Subtotal |
|
1 |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
3 (>0.02) |
|
|
Total |
Female |
87 |
288 |
624 |
1,506 |
738 |
71 |
68 |
7 |
3,389 (19.41) |
|
Male |
65 |
160 |
462 |
702 |
128 |
74 |
57 |
6 |
1,654 (9.47) |
|
Nymph |
701 |
864 |
1,368 |
388 |
172 |
604 |
40 |
0 |
4,137 (23.70) |
|
Larva |
5 |
5 |
16 |
85 |
3,230 |
4,246 |
685 |
5 |
8,277 (47.41) |
|
|
858 |
1,317 |
2,470 |
2,681 |
4,268 |
4,995 |
850 |
18 |
17,457 (100) |
Table 3Total number of hard ticks collected with a flagging method for 2 months in Ganghwa, 2015–2018
Table 3
|
Collection sites |
Date |
Species |
Number of collected hard ticks |
|
|
Larva |
Nymph |
Adult (Female) |
Adult (Male) |
Total |
|
Copse |
Jul. |
Haemaphysalis longicornis
|
|
208 |
100 |
45 |
|
|
|
H. flava
|
144 |
3 |
0 |
0 |
|
|
|
Ixodes nipponensis
|
|
0 |
0 |
0 |
|
|
Aug. |
H. longicornis
|
|
86 |
15 |
5 |
|
|
|
H. flava
|
2,861 |
0 |
0 |
0 |
|
|
|
Ix. nipponensis
|
|
2 |
0 |
0 |
|
|
Subtotal |
|
|
3,005 |
299 |
115 |
50 |
3,469 |
|
|
Short grass field |
Jul. |
H. longicornis
|
|
395 |
129 |
56 |
|
|
|
H. flava
|
102 |
3 |
0 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Aug. |
H. longicornis
|
|
116 |
25 |
4 |
|
|
|
H. flava
|
535 |
0 |
1 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Subtotal |
|
|
637 |
514 |
155 |
60 |
1,366 |
|
|
Coniferous forest |
Jul. |
H. longicornis
|
|
166 |
29 |
15 |
|
|
|
H. flava
|
70 |
4 |
0 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Aug. |
H. longicornis
|
|
84 |
11 |
6 |
|
|
|
H. flava
|
96 |
1 |
0 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Subtotal |
|
|
166 |
255 |
40 |
21 |
482 |
|
|
Broad-Leaved Forest |
Jul. |
H. longicornis
|
|
162 |
121 |
47 |
|
|
|
H. flava
|
104 |
4 |
5 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Aug. |
H. longicornis
|
|
37 |
33 |
13 |
|
|
|
H. flava
|
1,617 |
1 |
0 |
0 |
|
|
|
Ix. nipponensis
|
|
0 |
0 |
0 |
|
|
Subtotal |
|
|
1,721 |
204 |
159 |
60 |
2,144 |
|
|
Total (%) |
|
|
5,529 (74.11) |
1,272 (17.05) |
469 (6.29) |
191 (2.56) |
7,461 (100) |
References
- 1. Sonenshine DE, Roe RM. Biology of Ticks. 2:New York, USA. Oxford University Press. 2013.
- 2. de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci 2008;13:6938-6946.
- 3. Kovats S, Haines A. The potential health impacts of climate change: An overview. Med War 1995;11:168-178.
- 4. McMichael AJ, Woodruff RE, Hales S. Climate change and human health: Present and future risks. Lancet 2006;367:859-869.
- 5. Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol 2012;28:437-446.
- 6. Liu XY, Bonnet SJ. Hard tick factors implicated in pathogen transmission. PLoS Negl Trop Dis 2014;8:e2566.
- 7. Sajid MS, Kausar A, Iqbal A, Abbas H, Iqbal Z, Jone MK. An insight into the ecobiology, vector significance and control of Hyalomma ticks (Acari: Ixodidae): A review. Acta Trop 2018;187:229-239.
- 8. Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk of dengue in Europe. Acta Trop 2013;129:1-14.
- 9. Leger E, Vourc’h G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: causes and consequences. Exp Appl Acarol 2013;59:219-244.
- 10. Cumming GS. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology 2002;83:303-327.
- 11. Yu XJ, Liang MF, Zhang SY, Liu JD, Sun YL, Zhang QF, Popov VL, Li C, Qu J, Li Q, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Eng J Med 2011;364:1523-1532.
- 12. Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, Park C, Ryou J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick-borne Dis 2014;5:975-977.
- 13. Kim YH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 2013;19:1892-1894.
- 14. Liu K, Zhou H, Sun RX, Yao HW, Li Y, Wang LP, Di Mu, Li XL, Yang Y, Gray GC, Cui N, Yin WW, Fang LQ, Yu HJ, Cao WC. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci Rep 2015;5:9679.
- 15. Choi SJ, Park SW, Bae IG, Kim SH, Ryu SY, Kim HA, Jang HC, Hur J, Jun JB, Jung Y, Chang HH, Kim YK, Yi J, Kim KH, Hwang JH, Kim YS, Jeong HW, Song KH, Park WB, Kim ES. Severe fever with thrombocytopenia syndrome in South Korea, 2013–2015. PLoS Negl Trop Dis 2016;10:e0005264.
- 16. Korean Centers for Disease Control and Prevention. Disease Web Statistics System [internet]; Available from: http://iscdc.go.kr/dstar/jsp/stat/stat0001.jsp
- 17. Park SW, Han MG, Yun SM, Park C, Lee WJ, Ryou J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg Infect Dis 2014;20:1880-1882.
- 18. Gai Z, Zhang Y, Liang M, Jin C, Zhang S, Zhu C, Li C, Li X, Zhang Q, Bian P, Zhang L, Wang B, Zhou N, Liu J, Song X, Xu A, Bi Z, Chen S, Li D. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 2012;206:1095-1102.
- 19. Kim J, Bae JM. Epidemiological and clinical characteristics of confirmed cases of severe fever with thrombocytopenia syndrome in Jeju province, Korea, 2014–2018. J Prev Med Public Health 2019;52:195-199.
- 20. Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis 2012;12:156-160.
- 21. Bao CJ, Guo XL, Qi X, Hu JI, Zhou MH, Varma JK, Cui LB, Yang HT, Jiao YJ, Klena JD, Li LX, Tao WY, Li X, Chen Y, Zhu Z, Xu K, Shen AH, Wu T, Peng HY, Li ZF, Shan J, Shi ZY, Wang H. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: Further evidence of person-to-person transmission. Clin Infect Dis 2011;53:1208-1214.
- 22. Dobson ADM, Taylor JL, Randolph SE. Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: Hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick-borne Dis 2011;2:67-74.
- 23. Dominguez L, Miranda RJ, Torres S, Moreno R, Ortega J, Bermudez SE. Hard tick (Acari: Ixodidae) survey of Oleoducto trail, Soberania National Park, Panama. Ticks Tick-borne Dis 2019;10:830-837.
- 24. Köttek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z 2006;15:259-263.
- 25. Yamaguti N, Tipton V, Keegan H, Toshioka S. Ticks of Japan, Korea, and the Ryukyu Islands. 15:Provo, USA. Brigham Young University Science Bulletin. pp 1-227.
- 26. Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, Han MG. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis 2014;20:1358-1361.
- 27. Yun SM, Lee YJ, Choi WY, Kim HC, Chong ST, Chang KS, Coburn JM, Klein TA, Lee WJ. Molecular detection of severe fever with thrombocytopenia syndrome and tick-borne encephalitis viruses in ixodid ticks collected from vegetation, Republic of Korea, 2014. Ticks Tick-borne Dis 2016;7:970-978.
- 28. Shin J, Park J, Kwon D. Epidemiologic and clinical characteristics of severe fever with thrombocytopenia syndrome in the Republic of Korea. KCDC 2014;7:493-498.
- 29. Lee WT, Lim JW, Lee SY, Lee IY. Redistribution of Haemaphysalis flava and Ixodes tanuki collected from raccoon dog in Korea. Korean J Parasitol 1997;35:1-8.
- 30. Yun S, Song B, Choi W, Park W, Kim S, Roh J, Ryou J, Ju Y, Park C, Shin E. Prevalence of tick-borne encephalitis virus in Ixodid ticks collected from the Republic of Korea during 2011–2012. Osong Public Health Res Perspect 2012;3:213-221.
- 31. Min SK, Zhang X, Zwiers F, Shiogama H, Tung YS, Wehner M. Multimodel detection and attribution of extreme temperature changes. J Climate 2013;26:7430.
- 32. Yun SM, Song BG, Choi W, Roh JY, Lee YJ, Park WI, Han MG, Ju YR, Lee WJ. First isolation of severe fever with thrombocytopenia syndrome virus from Haemaphysalis longicornis ticks collected in severe fever with thrombocytopenia syndrome outbreak areas in the Republic of Korea. Vector Borne Zoonotic Dis 2016;16:66-70.
- 33. Wang S, Li J, Niu G, Wang X, Ding S, Jiang X, Li C, Zhang Q, Liang M, Bi Z, Li D. STFS virus in ticks in an endemic area of China. Am J Trop Med Hyg 2015;92:684-689.
- 34. Chae JB, Kang JG, Kim HC, Chong ST, Lee IY, Shin NS, Chae JS. Identification of tick species collected from wild boars and habitats of wild boars and domestic pigs in the Republic of Korea. Korean J Parasitol 2017;55:185-191.
- 35. Chae JB, Cho YS, Cho YK, Kang JG, Shin NS, Chae JS. Epidemiological investigation of tick species from near domestic animal farms and cattle goat, and wild boar in Korea. Korean J Parasitol 2019;57:319-324.
- 36. Chong ST, Kim HC, Lee IY, Kollars TM Jr, Sancho AR, Sames WJ, Klein TA. Comparison of dragging and sweeping methods for collecting ticks and determining their seasonal distributions for various habitats, Gyeonggi Province, Republic of Korea. J Med Entomol 2013;50:611-618.
- 37. Ham H, Jo S, Jang J, Choi S. No detection of severe fever with thrombocytopenia syndrome virus from Ixodid ticks collected in Seoul. Korean J Parasitol 2014;52:221-224.
- 38. Jo YS, Kang JG, Chae JB, Cho YK, Shin JH, Jheong WH, Chae JS. Prevalence of severe fever with thrombocytopenia syndrome virus in ticks collected from national parks in Korea. Vector Borne Zoonotic Dis 2019;19:284-289.