Dientamoeba fragilis Infection in Patients with Digestive and Non-Digestive Symptoms: A Case-Control Study
Article information
Abstract
In most developing countries, Dientamoeba fragilis infection is an obscure protozoan infection. We aimed to determine a frequency and clinical importance of D. fragilis infection in Taif, Saudi Arabia. A 1-year case control study included patients with gastrointestinal (cases, n=114) or non-gastrointestinal symptoms (controls, n=90). The fecal samples were examined with the classical parasitological methods for intestinal protozoa, and by real time PCR for D. fragilis. The infection by D. fragilis was detected in 5.8% by PCR and in 4.4% patients by microscopy. The infection was identified more in control group (n=9) than in cases (n=3); a sole infection in 11 patients and mixed with Giardia in 1 patient. The other enteric parasites detected were Blastocystis sp. (8.3%), Giardia sp. (5.3%), Cryptosporidium sp. (2.9%), Entamoeba histolytica (1.4%), Entamoeba coli (0.9%) and Hymenolepis nana (0.4%). Our results tend to reinforce the need to increase awareness of D. fragilis infection in Saudi Arabia.
INTRODUCTION
Dientamoeba fragilis is a single-celled protozoan parasite of the human gut. Infection may also be able to affect non-human primates and pigs. D. fragilis has emerged as a neglected cosmopolitan intestinal protozoa [1]. A prevalence of 0–82% has been recorded for the infection worldwide [2]. Most of these figures come from countries with high incomes. On the contrary, the prevalence of this infection in low-income countries is not well known [3]. The infection’s transmission and pathogenicity of D. fragilis remain a subject of an ongoing debate [4]. Fecal-oral transmission and infection along with pinworm eggs have often been suggested [5]. The parasite is often retrieved from patients with gastrointestinal symptoms [6]. Abdominal pain or cramps and diarrhea are common symptoms for Dientamoeba infection [7]. Other symptoms such as nausea, vomiting, anorexia, fever and eosinophilia have been also reported [8,9].
The classical parasitological methods (wet mount preparations and stool concentration techniques) make it difficult to detect D. fragilis in feces. Permanently staining methods or cultivation procedures are often required to achieve a correct parasitological diagnosis. This fact confirms that the nucleic acid-based assays have been the diagnostic method of choice for D. fragilis in feces [10].
In Saudi Arabia, infections with intestinal parasites pose a public health problem. Nonetheless, the diagnosis of these infections is largely done by microscopic examination of patients feces [11–13]. This strategy eventually led to numerous protozoan infections being overlooked, including D. fragilis. In this study, we aimed to assess incidence and clinical significance of D. fragilis infection in a clinic, Saudi Arabia.
MATERIALS AND METHODS
Ethical statements
This study got an approval (No: 41-710-0023) from the ethical committee of Applied Medical Sciences College at Al-Taif University. Recruitment of patients and collection of samples was carried out on a voluntary basis. Participants were given the research information and asked to sign a written consent form.
Study population
Patients visiting a primary health care clinic in Taif, Saudi Arabia, were invited to participate in a case-control study in 2018. Cases were chosen from patients with symptoms of enteric infections such as nausea, vomiting, urgency, loss of appetite, passage of bloody stool, passage of mucoid stool, and/or abdominal pain. Controls were selected from patients with non-gastrointestinal problems. Patients who have recently received anti-parasitic drugs and who have refused to provide fecal samples were excluded from this study.
Data collection
A questionnaire with specific demographic variables (age, gender, residence, recent travel to tropics and contact with infected household member) and clinical symptoms (diarrhea, abdominal pain or cramp, fever, nausea or vomiting, blood in stool or mucous in stool) was used in this study.
Parasitological examination
Stool samples (1 per patient) were obtained from all participants on the day of the interview or shortly afterwards. Fresh feces (within half an hour of collection) were microscopically screened for intestinal protozoa and instantly fixed; 1 part in 10% formalin and 1 part in 70% ethanol. Properly labeled preserved specimens were transported to the Medical Laboratory at Taif University’s College of Applied Medical Sciences. Smear preparations from formalin-fixed specimens were permanently stained inside the laboratory with iron-haematoxylin, Ziehl-Neelsen and trichrome stain, as mentioned elsewhere [14,15]. The ethanol-fixed specimens were kept at −20°C for Dientamoeba DNA detecting PCR.
RT-PCR for Dientamoeba fragilis
The ethanol preserved frozen feces were used for DNA extraction. Total genomic DNA was extracted and purified from stool specimen sediment (~200 mg) with QIAamp DNA Stool minikit (Qiagen, Hilden, Germany) following an amended manufacturer protocol [16]. Using a Dientamoeba fragilis-specific PCR, the fecal-recovered DNA was amplified and analyzed. The amplification reaction was initiated by a set of 1 pair of published primers (forward primer Df-124F: 5′-CAACGGATGTCTTGGCTCTTTA-3′, reverse primer Df-221R 5′-TGCATTCAAAGATCGAACTTATCAC-3′ and a TaqMan probe Df-172revT 5′-CAATTCTAGCCGCTTAT-3′ targeting 98 bp of 5.8 small subunits of the ribosomal RNA gene. The primers and the probe were synthesized by the VH Bio (Gateshead, UK). The reaction set up and thermal cycles were conducted in LightCycler (Roche Diagnostics Corporation, Mannheim, Germany). GoTaq Hot Start Polymerase (Promega) and other PCR reagents were used in amplification reactions with final concentrations closely similar to a published previous protocol [17].
Statistic analyses
In order to evaluate the qualitative variables of the study participants, the chi-square and Fisher’s exact test, on the Social Science Statistics website (https:/www.socscistatistics.com/) were implemented. P-value less than 0.05 was considered statistically significant. With aid of the MedCalc statistical software website (https:/www.medcalc.org/calc/diagnostictest.php), diagnostic performance of the permanently stain smear microscopy was calculated. The sensitivity, specificity, positive predictive value and negative predictive value of the permanently stained smear microscopy was calculated in comparison to the RT-PCR test’s results as a nominated gold standard.
RESULTS
We recruited 114 patients with gastrointestinal complaints (cases) and 90 patients with non-gastrointestinal complaints (controls) during the study period (Table 1). All matching conditions were met between cases and controls. The patients were presented with abdominal pain/cramp 39.2%, diarrhea 50% and nausea or vomiting 7.3%. Of the 204 samples tested, 50% showed diarrhea, 12.7% containing mucus and 4.9% bloody feces.
Dientamoeba fragilis trophozoites (Fig. 1) were detected from the stained smears of 9 patients, with a total prevalence of 4.4%. The RT- PCR detected D. fragilis DNA from 12 patients, 3 from cases and 9 from controls. Three fecal specimens found positive for the parasite DNA by the RT- PCR were missed by the microscopic examination. RT-PCR found all the microscopically positive samples. Taking the RT-PCR test results as a nominated gold standard, the sensitivity, specificity, negative and positive predictive values of the permanently stained smear microscopic examination were 75%, 100%, 98.4%, and 100%, respectively. Common symptoms associated with D. fragilis infection were abdominal pain 100%, diarrhea 66.6%, and vomiting 33.3%. All the D. fragilis-positive patients had chronic diarrhea and loose stool (Supplementary Table S1).

Microscopic image for Dientamoeba fragilis pleomorphic trophozoites in formalin-fixed iron-haematoxylin-stained fecal smear. Trophozoite having one nucleus and another with 2 nuclei (arrow).
The history of recent contact to a family member with gastrointestinal symptom(s) was reported less in cases than in controls, with observed significant difference (P<0.05). The infection was described in all age groups, but more patients in age category ≤19-year-old (10.8%). There was no statistically significant difference between cases and controls regarding D. fragilis positivity in all age groups. D. fragilis was described in 11 patients as a mono-parasitic infection and in 1 patient as coinfection with Giardia spp. This patient was an 11-year-old male child who had diarrhea for more than 2 weeks.
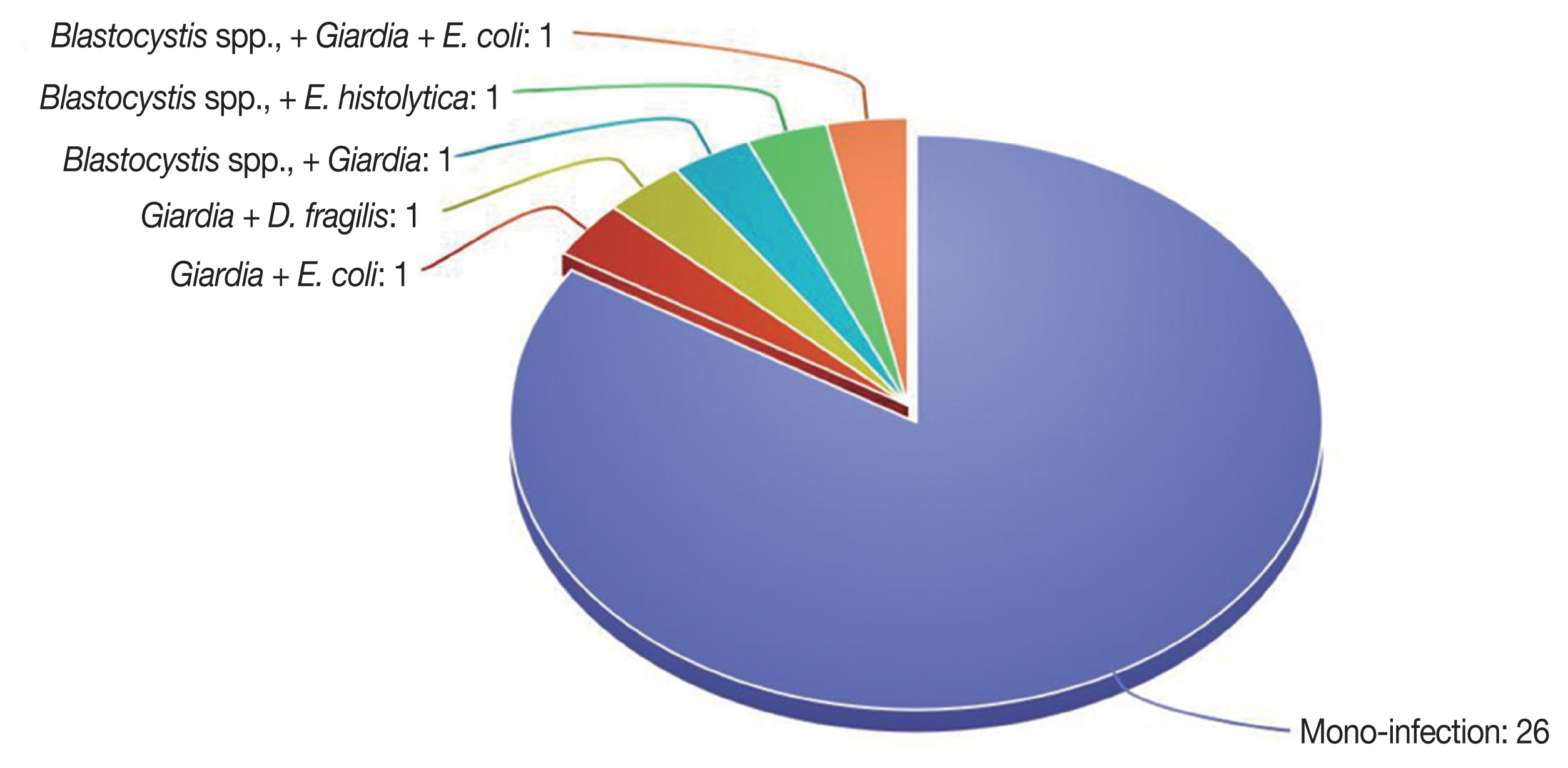
Other enteric parasites were detected (Table 2) from 31 cases and 4 controls with 17.1% total prevalence rate. There was significant difference between cases and controls for the enteric parasites’ positivity, (P<0.01). The enteric parasites included 8.3% Blastocystis sp., 5.3% Giardia sp., 2.9% Cryptosporidium sp., 1.4% Entamoeba histolytica, 0.9% Entamoeba coli, and 0.4% Hymenolepis nana. A significant difference regarding the positivity rates between cases and controls was observed for Blastocystis sp., Giardia and Cryptosporidium sp. Polyparasitism was reported in 5 cases (Fig. 2).
DISCUSSION
The D. fragilis infection is an unsolved issue in most developing countries and needs more focus. This was the first case-control study investigating the occurrence and the clinical significance of infection among a population from Saudi Arabia. A strength point, considered in our case-control study, was related to selection of the controls. We selected the control subjects from those attending a health care center with non-gastrointestinal symptoms to be representative for the same population as in cases to avoid selection bias. Another strength point considered in our study was related to the methodology adopted for D. fragilis detection. In this study, besides microscopy, we used a RT-PCR with sufficient sensitivity and specificity to enhance the detectability of the parasite in patients’ feces.
In the current study, D. fragilis was second to Blastocystis spp. Infection was detected with the RT-PCR at an estimated frequency rate of 5.8%. In Saudi Arabia, an estimate 0.2–0.6% of D. fragilis infection was described [18,19]. Wakid has identified the infection in food processing workers based on microscopic examination of permanently stain fecal smears. Microscopy was thought to be a less effective detection method for D. fragilis and often underestimated the frequency of the parasite, compared to the molecular diagnostic methods [20–22]. According to a recent study, there was an annual increase of 28% in the detection of D. fragilis infection after implementation of a fecal PCR in diagnosis. The RT-PCR used in our research was superior to permanently stain smear microscopy for diagnosis of D. fragilis in human [23,24]. Despite its high diagnostic sensitivity, due to high cost, many clinical laboratories, particularly those in poor countries, still hesitate to use PCR to detect D. fragilis in human feces. In this case, it is recommended to search for this protozoan infection through microscopic examination of permanently stained fecal smears by well-trained experts.
The occurrence and clinical importance of Dientamoeba infection in symptomatic and non-symptomatic individuals have been investigated in few case-control studies. The infection has been less frequently reported in patients with digestive symptoms than in asymptomatic individuals. In Holland, it was reported D. fragilis infection in 25.7% of symptomatic cases and 37.3% of asymptomatic control subjects [25]. In Denmark, the infection was 23% in patients and 35% in asymptomatic control subjects [26]. Here, in our research, we significantly identified D. fragilis in 2.6% of symptomatic cases and 10% of asymptomatic controls, coinciding with the above findings. The occurrence of high prevalence of D. fragilis in asymptomatic carriers was a surprising finding in our research. Such a finding raises the degree of uncertainty surrounding the pathogenic potential of the parasite in the population. In comparison to our results, there were also significantly higher prevalence rates (>20 percent) in several regions of the world, including countries from Europe, Middle East, and South America [27–29].
The common symptoms associated with D. fragilis infection were diarrhea, abdominal pain and vomiting, in agreement with the literature [30–33]. None of the Dientamoeba-positive patients, in our study, gave history of fever or reported blood or mucous in feces. The targeted population, method of detection used, infection severity, accompanying infections, and protozoan genotypes may affect the outcomes of infections in each study. In a recent study investigating the genetic diversity of Dientamoeba clinical isolates recovered from patients with irritable bowel syndrome, 4 distinct parasite subtypes have been identified, each type has been associated with different clinical presentations.
It was astonishing to us that concurrent infection with Giardia sp. was only 1 case. Dientamoeba/Giardia mixed infection was documented 7.1% from 1 population [33] and 9% from another [22] in disagreement with our study. Enteropathogens/D. fragilis coinfection was registered in 58% of a study population [35] and in 23% of another [22]. It is worth mentioning that the Blastocystis sp. has been the most common protozoan parasite cohabiting Dientamoeba infection [36]. Nonetheless, 1 recent report found that almost half of patients with dientamoebiasis was coinfected with Entamoeba histolytica/dispar [37]. Evidence about Dientamoeba infection acquisition along with pinworm infection have been reported [38], but still remains controversial. Enteropathogens other than the intestinal parasites like the enteroviruses and enteric bacteria were not sought for in the current survey study. These microbes could not be excluded as possible cohabiting pathogens with D. fragilis. One could not relate any symptoms to D. fragilis infection alone. In present study, colonization of D. fragilis was more common in patients aged 5–19 years [22]. Children of daycare-age or school-age and adults aged 30–40 years were 2 main age categories infected with D. fragilis [28].
In Saudi Arabia, Blastocystis sp., Giardia sp. and Cryptosporidium sp., were frequently detected. E. histolytica, E. coli and Hymenolepis nana were identified in few cases. The above parasitic infections were reported prevalent in Saudi communities [11,12,39].
In conclusion, our results tend to reinforce the need for increased awareness of D. fragilis infection in Saudi Arabia. The study described frequent D. fragilis infections in the asymptomatic controls, with reported RT-PCR superiority over microscopy in parasite’s detection.
ACKNOWLEDGMENTS
Authors would like to thank patients, health personnel, and technicians for their support and cooperation in this research work. The current study was supported by a special fund given from the Deanship of Scientific Research, Taif University, Saudi Arabia (Grant or Award Number 1-439-6081).
Notes
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this study.
Supplementary Materials
The demographic factors and clinical features of Dientamoeba-positives cases and controls