Molecular characterization of Acanthamoeba isolated from amebic keratitis related to orthokeratology lens overnight wear
Article information
Abstract
In an effort to characterize, on the molecular scale, the Acanthamoeba initially isolated from the cornea of an amoebic keratitis patient associated with overnight-wear orthokeratology lens in Korea, we conducted mitochondrial DNA restriction fragment length polymorphism, 18S rDNA sequencing, and drug sensitivity analyses on the isolate (KA/PE1). The patient was treated with polyhexamethylene biguanide, chlorhexidine and oral itraconazole, which resulted in resolution of the patient's ocular inflammation. The majority of the molecular characteristics of the KA/PE1 were determined to be identical, or quite similar, to those of A. castellanii Ma strain, which had been isolated also from amoebic keratitis. The risk of Acanthamoeba keratitis as a potential complication of overnight orthokeratology is briefly discussed.
INTRODUCTION
Acanthamoeba has been noted as a relatively uncommon causative organism of chronic infectious keratitis in contact lens users, since the first case of Acanthamoeba keratitis was reported in 1975 (Jones et al., 1975). The number of Acanthamoeba keratitis cases is rapidly increasing over the recent years.
Orthokeratology is targeted toward the temporary reduction of myopia and astigmatism via the flattening of the corneal curvature (Wilson and Keeney, 1990). These reverse-geometry and rigid gas-permeable contact lenses are worn overnight, thereby allowing the patient to achieve improved uncorrected visual acuity during the day (Alharbi and Swarbrick, 2003). Although the advent of this reverse-geometry contact lens design, coupled with novel rigid gas-permeable materials evidencing high oxygen transmissibility, contribute to more stable and safer effects, the inherent tight bending of the cornea and overnight wear have both been identified as risk factors for infectious keratitis (Dart, 1998; Nichols et al., 2000; Chen et al., 2001). Many reported cases of orthokeratology-associated microbial keratitis have been caused by Pseudomonas aeruginosa (Lau et al., 2003; Young et al., 2003, 2004), but Acanthamoeba is becoming recognized as one of the primary causes, as well (Xuguang et al., 2003; Hutchison and Apel, 2002; Wilhelmus, 2005).
In any determination of the ecology and prevalence of pathogenic strains of Acanthamoeba, the species identification of the ocular isolates is a prerequisite. Although more than 20 species of Acanthamoeba have so far been classified largely into 3 groups in accordance with their morphological characteristics (Pussard and Pons, 1977), the taxonomy of this small ameba species has yet to be thoroughly established. Alloenzyme profiles and mitochondrial DNA restriction fragment length polymorphism (mtDNA RFLP) studies have been extensively conducted, but the obtained results were too polymorphic to constitute criteria for species identification (Kong et al., 1995; Chung et al., 1996). Recently, riboprinting and 18S rDNA gene sequence analyses have been applied to species identifications of ameba strains (Gast et al., 1996; Chung et al., 1998; Stothard et al., 1998).
The present study describes the results of molecular characterization of the first ocular isolate (KA/PE1), originated from an amebic keratitis patient associated with overnight-wear orthokeratology lenses in Korea, as well as the results of our comparison of mtDNA RFLP and 18S rDNA sequencing analyses among the isolates and reference strains.
MATERIALS AND METHODS
Case
A 15-year-old girl who had been wearing orthokeratology lenses for 10 mo was referred to our hospital complaining of severe ocular pain and photophobia on both eyes, which had apparently persisted for 1 week despite the patient's use of antibacterial eye drops and ointment. The patient was employing multipurpose solution on a regular basis, and denied having rinsed her contact lenses with tap water. Her right eye visual acuity was 15/100 and left eye was measured at 10/100. Radial keratoneuritis coupled with severe conjunctival injection was detected in both eyes (Figs. 1A & 1C). Corneal scraping revealed an amebic cyst via histopathological evaluation. Acanthamoeba (KA/PE1) trophozoites grew out from corneal scraping, swab of contact lens, and pellet of multipurpose solution on 1.5% non-nutrient agar plate covered with heat inactivated E. coli. The patient was treated at hourly intervals with polyhexamethylene biguanide (PHMB) 0.02%, chlorhexidine 0.02%, which were gradually tapered. Oral itraconazole was administered for 10 days. After 6 mo, the cornea appeared clear with no impairment, and the best-corrected visual acuity was 20/20 in both of the patient's eyes (Figs. 1B & 1D).
Axenization and cultivation
A piece of agar plate (0.5 × 1 cm) covered with KA/PE1 cysts was treated for 24 hr with 0.1 N HCl for axenization, and then washed 3 times in sterile water. The agar plate was placed in PYG or PYGC media (Kong et al., 1995) and incubated at 25℃. The KA/PE1 cysts were measured for size, and the number of arms was determined for the purpose of morphological grouping.
Four reference strains, A. castellanii Castellani, A. castellanii Ma, Acanthamoeba sp. KA/E1 and Acanthamoeba sp. KA/E2 were also cultured in PYG media at 25℃.
Extraction of mtDNA and RFLP
The mtDNA of KA/PE1 was extracted in accordance with the method described by Yagita and Endo (1990). After then, it was digested with 4 restriction enzymes (Eco RI, Bgl II, Sca I, and Cla I) and other reference strains using 2 types of restriction enzymes (Eco RI and Bgl II), for 4 hr at 37℃ in a 20 µl reaction volume with the buffers specified for each of the restriction enzymes. The digested DNA was then electrophoresed for 1-2 hr in 0.7% agarose gel at 4 V/cm and stained for 15 min with ethidium bromide. The mtDNA RFLP patterns were then visualized and photographed with an UV transilluminator.
Chromosomal DNA extraction, 18S rDNA amplification and sequencing
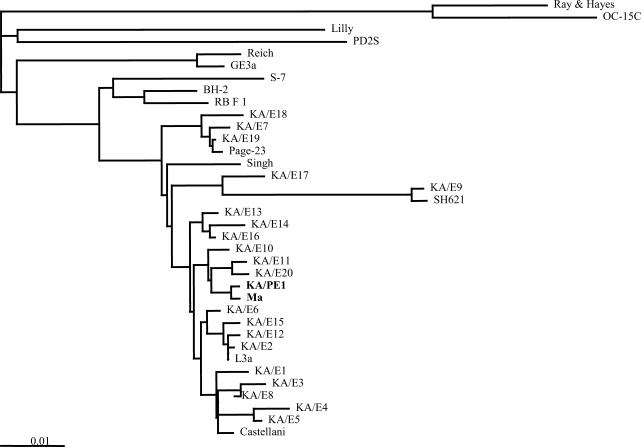
The chromosomal DNA of KA/PE1 was acquired in accordance with the method developed by Kong and Chung (1996). The DNA was then utilized as a PCR template. The primers, P3; 5'-CCG AAT TCG TCG ACA ACC TGG TTG ATC CTG CCA GT-3', P4; 5'-GGA TCC AAG CTT GAT CCT TCT GCA GGT TCA CCT AC-3', were designed to hybridize to highly conserved sequences at the extreme 5' (P3) and 3' (P4) termini of the eukaryotic 18s rDNA (Chung et al., 1998). The PCR was conducted using a premixed PCR reagent kit (Bioneer, Daejeon, Korea) and a thermal cycler (Perkin Elmer Cetus, Foster, California, USA). The PCR products were sequenced with the Gibco BRL dsDNA Cycle Sequencing System (Life Technologies, Gaithersburg, Maryland, USA), either directly or after cloning into pBSK+ (Stratagene, La Jolla, California, USA) or PCR II (Invitrogen, San Diego, California, USA). The sequence was then compared with those of other Acanthamoeba species, which are available in the GenBank reference strain database (Table 1). A phylogenetic tree was reconstructed via the Neighbor-Joining method.
In vitro drug sensitivity
We assessed the drug sensitivity of the cyst forms of KA/PE1, in accordance with the method developed by Yu et al. (2004). In brief, the KA/PE1 cysts were recovered from the E. coli plates, washed, and adjusted to a concentration of 5 × 103/100 µl. A cyst suspension (100 µl) was then inoculated on a 96-well microplate, and each of the wells was treated with from 2-fold serial dilutions of chlorhexidine, PHMB, and hexamidine (initial concentration of all drugs was 200 µg/ml). The minimal cysticidal concentration (MCC) of each disinfectant was defined as the minimal concentration of test solution resulting in no excystment or trophozoitic replication after 7 days. The MCC was calculated after 3 consecutive experiments.
RESULTS
Morphology Cysts of the clinical isolate, KA/PE1, evidenced morphological characteristics typical of the Pussard and Pons (1977) morphological group 2, characterized by satellite or polygonal endocysts and wavy ectocysts (Fig. 2). They were found to have almost identical sizes in terms of cyst diameter (14.0 to 17.0 µm; average 15.2 µm) and the number of arms (4 to 8; average 5.5).
Drug sensitivity
KA/PE1 was extremely sensitive to chlorhexidine (MCC; 12.5 µg/ml) and PHMB (MCC; 6.25 µg/ml) treatments for 8 hr, but 8 hr of hexamidine treatment proved ineffective.
mtDNA RFLP and 18S rDNA sequence analyses
KA/PE1 evidenced mtDNA RFLP patterns identical to those of a clinical isolate of A. castellanii Ma (USA) and an Acanthamoeba sp. KA/E1 Korean clinical isolate via Eco RI and Bgl II restriction enzymes (Fig. 3).
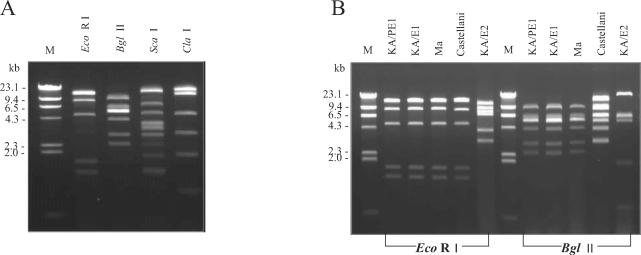
Agarose gel electrophoretic restriction fragment patterns of the mtDNA of an Acanthamoeba clinical isolate, KA/PE1 (A), and 4 reference strains (B). M, Hind III digested lambda phage DNA as a DNA size standard; mt DNA RFLP patterns by Eco RI and Bgl II of KA/PE1 were identical to those of A. castellanii Ma.
The PCR product for 18S rDNA of this isolate was determined to be approximately 2,300 bp. A phylogenetic tree predicated on the 18S sequence data indicates that Acanthamoeba sp. KA/PE1 was quite closely related to A. castellanii Ma, Castellani, A. lugdunensis L3a, and the majority of the Korean clinical isolates. On the basis of the sequence homologies with A. castellanii Ma, the mtDNA pattern of RFLP KA/PE1 indicated its identification to be A. castellanii Ma.
DISCUSSION
Acanthamoeba keratitis is an infection that has been strongly associated with the use of contact lenses. Recently, many of the usual complications of contact lenses have been shown to be applicable to orthokeratology, and corneal infection is probably the most serious complication associated with wearing of contact lenses (Xuguang et al., 2003). Orthokeratology lenses are designed to flatten the central cornea. Their base curve tends to be flatter than the anterior corneal curvature. Thus, alterations in the cornea, including corneal epithelial edema, abrasion, and corneal staining can occur, and can predispose the central cornea to an increased risk of infection (Nichols et al., 2000).
Orthokeratology lenses, when worn overnight, provide a potential stimulus to this abrasion of microtrauma (Hutchinson and Apel, 2002). Therefore, the mechanism underlying orthokeratology-related infectious keratitis is likely to involve a combination of altered corneal defenses, including microtrauma, hypoxia, and adherence of microorganisms to the corneal epithelium. Although recent advances in high-Dk materials and the new generation of reverse-geometry design have resulted in more stable and safe orthokeratology, these advances have not eliminated infectious keratitis as a complication of orthokeratology (Yepes et al., 2005).
Recently, many cases of orthokeratology-associated infectious keratitis have been reported, including Acanthamoeba-mediated cases (Hutchison and Apel, 2002; Xuguang et al., 2003; Wilhelmus, 2005). Acanthamoeba infections are associated with the presence of corneal abrasion, the binding of the parasite to the cornea and contact lenses, and improper use of cleaning and disinfecting solutions. We suggest that Acanthamoeba keratitis may become a sight-threatening complication associated with overnight orthokeratology in cases in which risk factors exist, in particular contaminated water.
Clinically, the features of Acanthamoeba infection-associated keratitis resemble those observed with Herpes simplex or, in some instances, with infections with bacteria or fungi (Kirkness et al., 1994). These similarities often result in inappropriate medical treatment; subsequent Acanthamoeba culture isolation can be rendered more difficult under such circumstances. Delayed diagnosis or misdiagnosis as bacterial or Herpes simplex keratitis can result in extensive corneal inflammation and profound visual loss. Therefore, accurate and rapid diagnoses of Acanthamoeba keratitis are essential for successful treatment and favorable prognoses.
Successful medical treatment is the objective in terms of the management of this disease, thereby circumventing the requirement for corneal transplantation. It is our experience, and that of other experts, that Acanthamoeba-associated keratitis diagnosed at earlier stages can be more successfully medically treated, without resorting to keratoplasty in an uncontrolled infection situation (Larkin et al., 1992). With the increased awareness of Acanthamoeba keratitis among clinicians and the availability of rapid diagnostic techniques, this infection is, indeed, being diagnosed at earlier stages. Laboratory diagnosis is primarily conducted via culture of epithelial scraping samples inoculated onto non-nutrient agar plates spread with bacteria. Direct microscopy of samples may also be employed, using stains for the cyst wall or immunostaining. A variety of topically applied therapeutic agents, including propamidine isethionate, clotrimazole, PHMB, and chlorhexidine, are believed to be effective. As demonstrated in our study, early diagnosis and prompt initiation of PHMB treatment could render the visual outcome of Acanthamoeba keratitis favorable (Duguid et al., 1997).
Although it is unlikely that species identification of Acanthamoeba isolates from clinical samples is critical for chemotherapy, genetic characterization is necessary for understanding molecular epidemiology (Ledee et al., 1996). In the past, 18S rDNA sequence analysis was considered to be the proper method for identification of unknown Acanthamoeba isolates from clinical and environmental samples (Stothard et al., 1998; Kong et al., 2002; Yu et al., 2004). Acanthamoeba isolates, the 18S rDNA sequences of which were closely associated with A. castellanii Ma, have already been shown to induce human amebic keratitis in Japan (Ma et al., 1981; Yagita et al., 1990). However, this is the first report regarding amebic keratitis related to wearing of overnight orthokeratology lenses in Korea, the identification of which was predicated on molecular characteristics. A. castellanii has been most frequently isolated from contact lens storage cases in Korea (Lee et al., 1997; Yu et al., 2001; Kong et al., 2002; Jeong and Yu, 2005).
Orthokeratology is frequently prescribed to children in cases such as the one in our study, in an attempt to slow down the progression of myopia. Parents of children who are considering overnight orthokeratology should evaluate carefully the benefits of temporary myopia reduction, as well as the risk of infection. In addition, they should acquire as much information as possible regarding infectious keratitis. In order to prevent Acanthamoeba-associated keratitis, attention should be paid to the hygienic maintenance of orthokeratology lenses in Korea.
References
Notes
This work was supported for two years by Pusan National University Research Grant.