Current Knowledge of Small Flukes (Digenea: Heterophyidae) from South America
Article information
Abstract
Fish-borne heterophyid trematodes are known to have a zoonotic potential, since at least 30 species are able to infect humans worldwide, with a global infection of around 7 million people. In this paper, a ‘state-of-the-art’ review of the South American heterophyid species is provided, including classical and molecular taxonomy, parasite ecology, host-parasite interaction studies and a list of species and their hosts. There is still a lack of information on human infections in South America with undetected or unreported infections probably due to the information shortage and little attention by physicians to these small intestinal flukes. Molecular tools for specific diagnoses of South American heterophyid species are still to be defined. Additional new sequences of Pygidiopsis macrostomum, Ascocotyle pindoramensis and Ascocotyle longa from Brazil are also provided.
INTRODUCTION
The Opisthorchioidea Looss, 1899 (Digenea) comprises a group of species of medical and veterinary importance with a worldwide distribution for which approximately 100 life cycles have been described [1–6].
Heterophyids are usually parasitic as adults in mammals and birds, and utilize mollusks and then generally fishes as intermediate hosts. Such fish-borne heterophyids are known to have a zoonotic potential. According to Chai and Jung [7], 30 species are able to infect humans worldwide, with a global level of infection of around 7 million people. Integrative studies, including molecular data associated with structural, morphological and ecological aspects, have helped elucidate the taxonomy and phylogeny of the group [6], although, based on limited molecular evidence, Thaenkham et al. [8,9] have questioned the recognition of this family as distinct from the Opisthorchiidae. It is clear that further taxonomic studies of the Heterophyidae are required, since recognition is the basis for prevention and control programs for these emerging fish-borne trematodoses [10]. Not only taxonomic problems but also other dubious aspects of the biology of these parasites need to be solved via the use of molecular tools [11]. According to Chai and Lee [12], of the approximately 70 species of intestinal trematodes that parasitize humans, more than 30 belong to Heterophyidae. Humans acquire these fish-borne zoonotic trematode (FZT) infections by eating raw or inadequately cooked fish. The main symptoms include abdominal pain, flatulence, diarrhea, eosinophilia, lethargy and anorexia. Occasional severe symptoms or even deaths have been observed when heterophyid eggs reach the blood or lymphatic vessels and migrate to the cardiac muscle and brain [1,12].
In this paper we present a review of the research on South American (SA) heterophyid trematodes.
TAXONOMY AND LIFE CYCLES
The genus Ascocotyle Looss, 1899 is represented by numerous species composing the so called “Ascocotyle complex” [13,14]. The distribution of those species in different genera and subgenera represented a problem in the taxonomy of the group. The most commonly used classification was based on Sogandares-Bernal and Lumsden [13] which considered Ascocotyle subdivided into 3 sub-genera: Ascocotyle (Ascocotyle), Ascocotyle (Leighia), and Ascocotyle (Phagicola). The subgenera Ascocotyle and Leighlia included adults with 2 rows of oral spines and vitellarium extending up to the ventral sucker; the former had the uterus confined posterior to ventral sucker and a parapleurolofocercous-type cercaria while in the latter the uterus extended to the pharynx level and had ophthalmogymnocephalous-type cercaria. The subgenus Phagicola comprised adults with 1, 2 or no rows of oral spines, vittelarium extending only to the ovary level and the cercaria was of pleurolofocercous type [13].
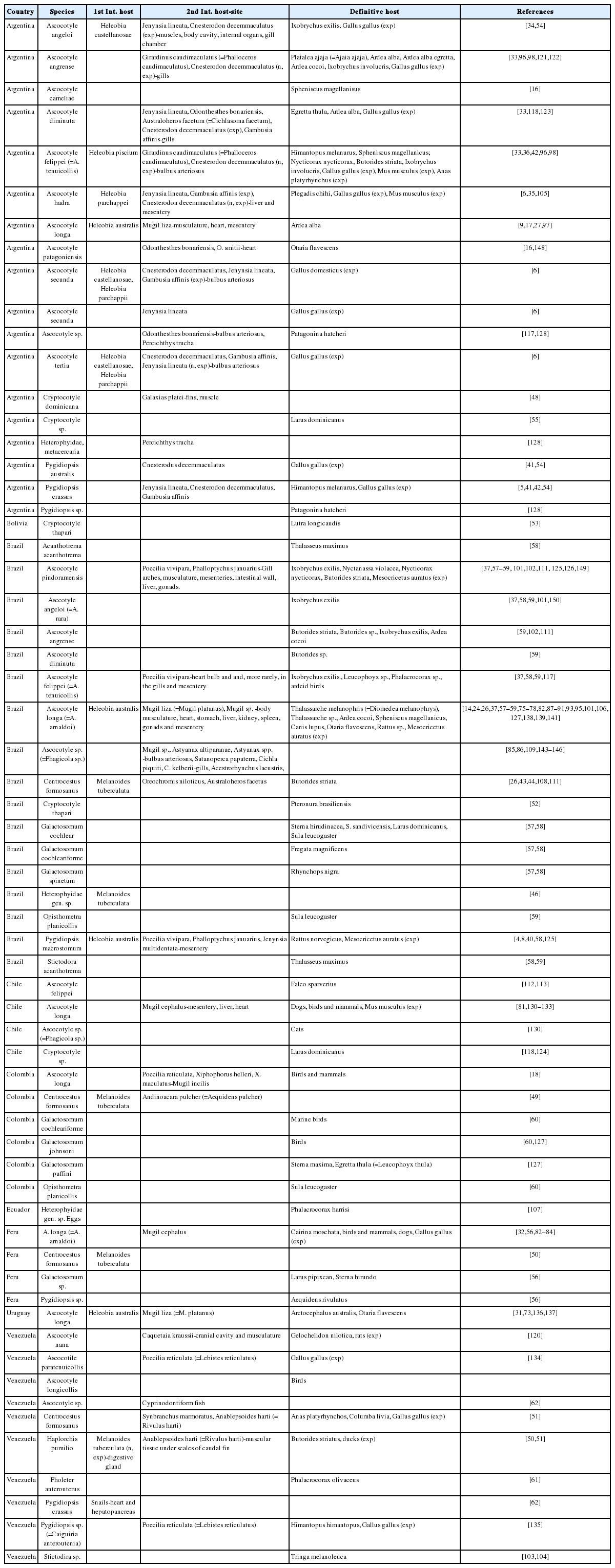
Pearson [15] revised the family Heterophyidae and did not recognize subfamilies or subgenera, considering the following genera occurring in SA: Acanthotrema Travassos, 1928, Ascocotyle Looss, 1899 (syn. Phagicola Faust, 1920; Parascocotyle Stunkard & Haviland, 1924; Metascocotyle Ciurea, 1933; Pseudascocotyle Sogandares-Bernal & Bridgman, 1960; Leighia Sogandares-Bernal & Lumsden, 1963); Centrocestus Looss, 1899, Cryptocotyle Lühe, 1899, Heterophyes Cobbold, 1886, Opisthometra Poche, 1926, Pholeter Odhner, 1914, and Pygidiopsis Looss, 1907 (syn. Caiguiria Nasir & Diaz, 1971). Several authors continued using the subgenera of Ascocotyle [16–18]. The WoRMS database [19] followed Pearson’s taxonomy [15] and authors tend to avoid using subgenera for the stability of the taxa. The current list of species occurring in SA with their hosts and geographical distribution is presented in Table 1. The host names are based on ITIS online database [20].
The current list of species occurring in South America with their hosts and geographical distribution
Species of the genus Ascocotyle are the most prevalent in SA and include Ascocotyle angeloi Travassos, 1928 (syn. A. rara), Ascocotyle angrense Travassos, 1916, Ascocotyle cameliae Hernández-Orts, Georgieva, Landete & Scholz, 2019, Ascocotyle diminuta (Stunkard & Haviland, 1924), Ascocotyle felippei Travassos, 1928, Ascocotyle hadra Ostrowski de Núñez, 1992, Ascocotyle longa Ransom, 1920, Ascocotyle patagoniensis Hernández-Orts, Montero, Crespo, García, Raga & Aznar, 2012, Ascocotyle pindoramensis (Travassos, 1928), Ascocotyle secunda Ostrowski de Núñez, 2001 and Ascocotyle tertia Ostrowski de Núñez, 2001.
Out of these, a species considered as causing an emerging fish-borne disease in humans is A. longa, a parasite recorded throughout the Americas, Europe, Africa and the Middle East [1,21–25]. Its life-cycle includes adult parasites in the intestine of fish-eating birds and mammals and the metacercaria mainly in mullets (Mugil spp.) [14,18,24,26–31]. The life cycle of A. longa is associated with estuaries and coastal lagoons, where the mugilid juveniles become infected [7,17,24,26–28,30]. The first intermediate host in Argentina, Brazil and Uruguay is the snail Heleobia australis (d’Orbigny, 1835), in which rediae and parapleurolophocercous cercariae develop [7,31]. Metacercariae have been found encysted in the body musculature, heart, stomach, liver, kidney, spleen, gonads and mesentery of the mullets [17,26,27,32]. Galván-Borja et al. [18] examined mullets from Colombia and reported that these metacercariae cause inflammatory reactions involving macrophage aggregates and necrosis, thus suggesting that trematodes infecting the liver of mugilids can affect the growth and well-being of the fish. The life-cycles of A. diminuta, A. angrense, A. hadra, A. felippei, A. secunda and A. tertia were studied in Argentina and follow similar pattern to that of A. longa and the adults mainly parasitize fish-eating birds and mammals [6,33–36] (Table 1).
Three species of Pygidiopsis have been reported in SA: Pygidiopsis macrostomum Travassos, 1928, Pygidiopsis crassus Ostrowski de Núñez, 1995 and Pygidiopsis australis Ostrowski de Núñez, 1996. Pygidiopsis macrostomum was originally described based on a single specimen obtained from the intestine of Rattus norvegicus (Erxleben, 1777) in Brazil [37], but was subsequently reported from the intestine of the bulldog-bat Noctilio leporinus mastivus Ribeiro, 1914 in Cuba [38,39]. This parasite was redescribed using morphological, ultrastructural and molecular data [8,40], and its life cycle was completed using naturally infected snails and fish, and hamsters as experimental definitive hosts [4]. The snail H. australis acts as the first intermediate host, the fishes Phalloptychus januarius (Hensel, 1868), Jenynsia multidentata (Jenyns, 1842) and Poecilia vivipara (Bloch & Schneider, 1801) as second intermediate hosts, and fish-eating mammals and birds as definitive hosts [4]. Indirect immunocytochemistry and phalloidin-fluorescence techniques, allied with confocal laser scanning microscopy, were also used by Borges et al. [8] to describe the muscular and neuronal structures of Pygidiopsis macrostomum, revealing the complex arrangement of muscular fibers and ganglia within the body. Pygidiopsis crassus was reported from Argentina and Venezuela while Pygidiopsis australis only from Argentina; metacercariae were found in small fish, and adults in Himantopus melanurus (Viellot, 1817) and experimentally infected Gallus gallus (Linnaeus, 1758) [5,6,41,42].
Centrocestus formosanus Nishigori, 1924, a species considered of zoonotic importance, was introduced in the Americas within its host snail Melanoides tuberculata. It has been reported from Brazil [43–47], Colombia [48], Peru [50] and Venezuela [51]. The life-cycle includes naturally infected fish (metacercariae in the gills) and birds (adults in the intestine) and experimental infections were performed with G. gallus [45] (Table 1). To our knowledge there are no reports on human infections by C. formosanus in SA.
Cryptocotyle thapari McIntosh, 1953 described from the otter Pteronura brasiliensis Zimmermann, 1780 from U.S. National Zoo in Washington, D.C. was later redescribed from specimens collected from Lontra longicaudis (Olfers, 1818) (=Lutra longicaudis) from Bolivia [52,53]. Cryptocotyle dominicana Casalins, Arbetman, Viozzi & Altman, 2020 was described from Larus dominicanus Lichtenstein, 1823, and the metacercariae from Galaxias platei Steindachner, 1898 were conspecific based on morphometrics and genetic markers [48].
Few records of Galactosomum spp. have been reported from Brazil, Colombia and Peru from marine birds [54–58]. Opistometra planicollis (Rudolphi, 1819) was only recorded from the same bird Sula leucogaster (Boddaert, 1783) in both countries [59,60]. Some species have been only reported from Venezuela, such as Haplorchis pumilio (Looss, 1896), Pholeter anterouterus Fischtal & Nasir, 1974 and Stictodora sp. Pholeter anterouterus and Stictodora sp. were reported parasitizing birds while H. pumilio considered an introduced species parasitizes the introduced M. tuberculata, the fish Anablepsoides harti and the bird Butorides striatus, and experimentally infected ducks [50,54,61–63] (Table 1).
MOLECULAR STUDIES
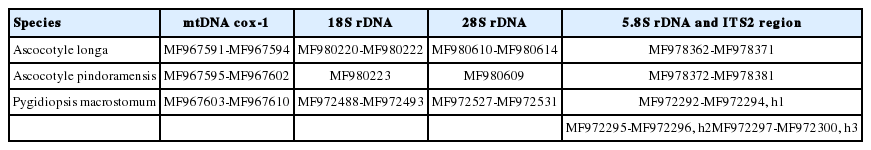
Sequences of the 18S, 28S, and ITS2 rDNA regions of Pygidiopsis macrostomum and mtDNA cox-1 sequences of Ascocotyle pindoramensis have already been described [8]. Molecular phylogeny linked P. macrostomum and A. pindoramensis with other heterophyids but, in agreement with Thaenkham et al. [10], the distinction of the Heterophyidae and Opisthorchiidae remained unclear. Additionally, new sequences of P. macrostomum, A. pindoramensis and A. longa from the nuclear regions 18S, 28S, and ITS2 rDNA and the mitochondrial marker mtDNA cox-1 are hereby presented and deposited in GenBank (Table 2), following the methodology of Borges et al. [8]. The sequence analysis of the 18S and 28S rDNA regions of these species did not exhibit any intraspecific variability, but some variability was observed between the species. BLAST analyses indicated a high similarity with other heterophyiid genera and species deposited in GenBank. Concatenated phylogenetic analyses (18S and 28S) readily differentiated the heterophyid genera Metagonimus, Haplorchis and Procevorum (Fig. 1), which demonstrates that these gene regions can be useful for solving low-level taxonomic problems when using concatenated genes with higher evolutionary rates. Martorelli et al. [17], Dzikowski et al. [64] and Alda et al. [9] sequenced the 18S rDNA region of A. longa from Israel and Argentina, the sequences of which are deposited in GenBank under the accession numbers AY245703, JX093559, and KF697717, respectively. The comparison of the genetic variability of 18S rDNA among these and our sequences of A. longa (MF980220 and MF980222) resulted in high values of the p distance ranging from 1.6 to 3.3%. This indicates the necessity of a wider, more comprehensive study of A. longa, comparing samples from different geographical regions in order to verify the existence of a complex of sibling species.
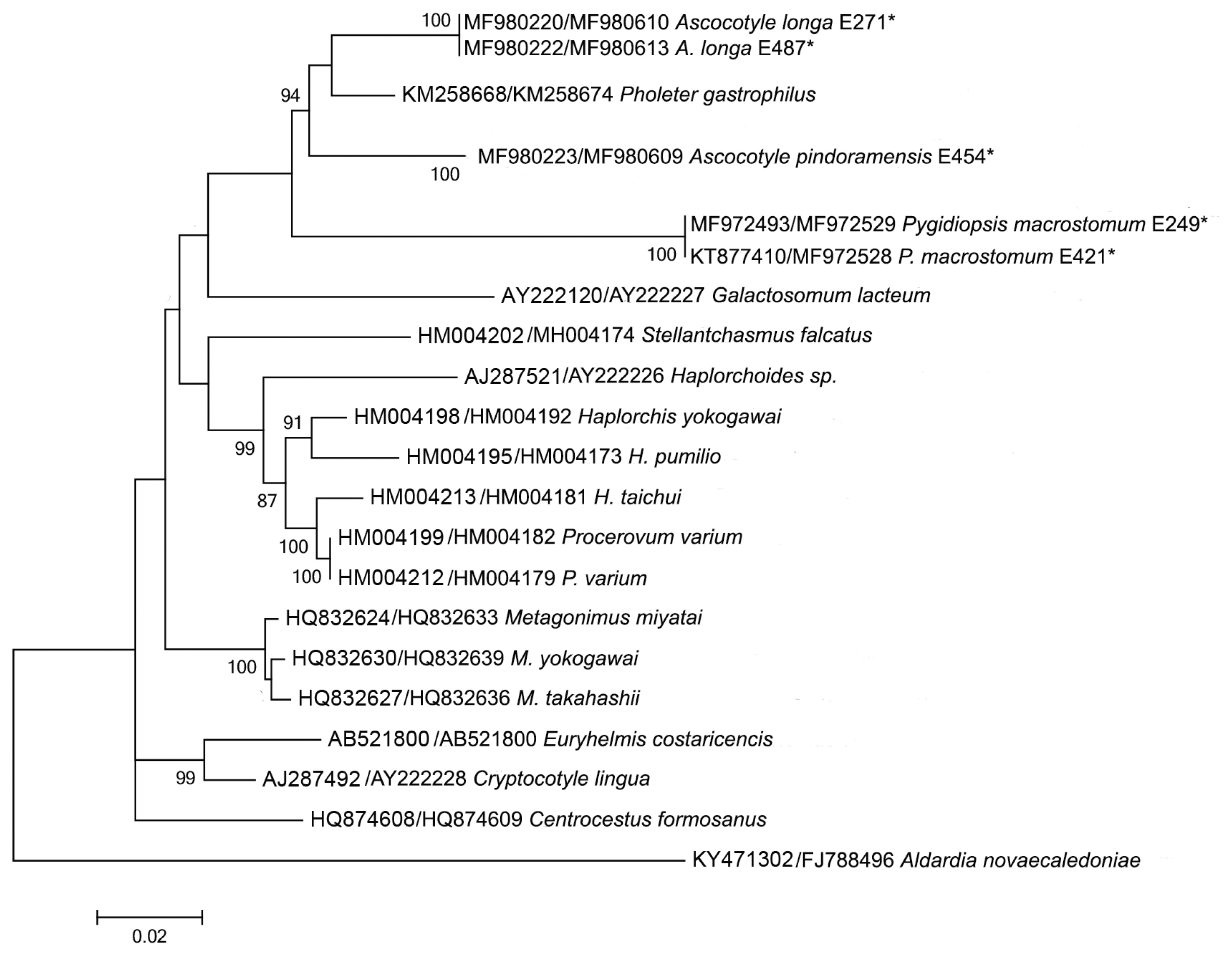
Maximum likelihood reconstruction between sequences obtained by our research group (marked with an asterisk) and sequences of heterophyid species from the GenBank database, with the tree inferred from 18S rDNA and 28S rDNA data sets. The numbers on the tree branches represent the percentage of bootstrap resampling.
The comparison of ITS2 rDNA sequences of P. macrostomum resulted in 3 haplotypes with an intraspecific p distance ranging from 0.4% to 1.9% between them. Such a level of variability appears to be acceptable according to Miller and Cribb [65] who reported 5% variability among species of heterophyids. However, a comparison of new sequences of the ITS2 rDNA of A. longa and A. pindoramensis did not result in any intraspecific variation. Similar results with heterophyids of the genus Haplorchis were reported [66] without intraspecific variability using the ITS2 rDNA region. The intrageneric distance, based on sequences of the ITS2 rDNA of the species A. longa and A. pindoramensis, was as high as 12.8%, indicating that the ITS2 region is a good marker for identifying closely related species. According to Brusentsov et al. [67], genetic variability provides species survival and resilience to ecosystems.
Phylogenetic analysis carried out using the mtDNA cox-1 region resulted in poorly supported branches, which may indicate a lack of phylogenetic signal of this marker for comparing species from higher taxa, such as at the family level (Fig. 2). Vanhove et al. [68] and Besansky et al. [69] discussed the problematic use of generic primers for highly variable regions, such as the mtDNA cox-1, for elucidating taxonomic relationships between platyhelminth families, proposing the use of 28S rDNA and ITS rDNA regions as more reliable barcodes for this phylum.
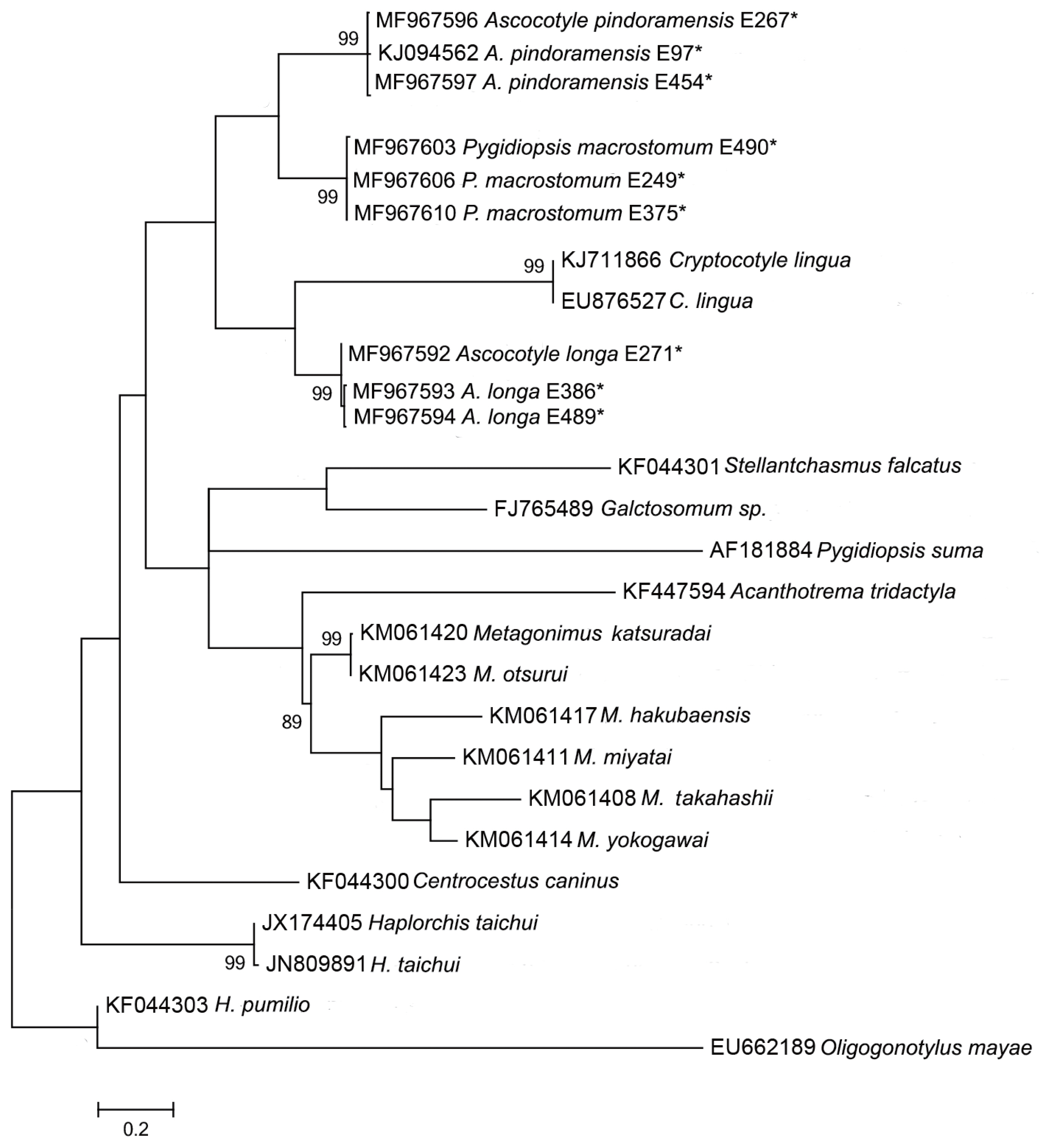
Maximum likelihood reconstruction between sequences obtained by our research group (marked with an asterisk) and sequences of heterophyid species from the GenBank database, with the tree inferred from the mtDNA-cox1 data set. The numbers on the tree branches represent the percentage of bootstrap resampling.
BEHAVIOR AND ECOTOXICOLOGY
The influence of the metacercariae of A. pindoramensis on the behavior of the fish-host Poecilia vivipara was investigated using an image system linked to a video camera able to record the locomotory activity of the fishes before and after experimental infections [70]. The results indicated a significant decrease in the swimming behavior of fish after 14 days post-infection when metacercariae were fully developed, correlated with parasite intensity [70].
The heterophyid trematode C. formosanus was introduced into Brazil with the gastropod Melanoides tuberculata, which has a wide geographic distribution in the Neotropics [47]. This species, which is considered to have zoonotic potencial, is able to alter the locomotory activity of its snail host regardless of the standard length [47].
Ecotoxicological and behavioral experiments with heterophyids were performed in order to study the effect of cyanobacteria on both the parasite (metacercaria) and its fish host. The fish Poecilia vivipara is a common host of Pygidiopsis macrostomum off the coast of Rio de Janeiro. This fish-trematode interaction was tested as a model for ecotoxicological studies with the cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) [71,72]. Changes in the motility of metacercariae of P. macrostomum occurred after their fish host was exposed to different concentrations of crude lyophilized extract of the cyanobacterium CYRF-01 producer of neurotoxic saxitoxins (STX), resulting in a temporary paralysis [72]. Interestingly, their motility recovered after the fish were kept for 48 hr in clean water, suggesting that blooms of cyanobacteria may interfere with the motility of both the fish host and its parasites.
TREMATODOSIS
Ascocotyle longa has a wide geographic distribution, comprising the Mediterranean Sea and the coasts of the North Atlantic, South Atlantic and Pacific [73]. In SA, it has been reported parasitizing different mugilid fishes from Venezuela, Peru and Brazil [73–76]. This species has also been reported as parasitizing avian hosts [77,78] and dogs in the USA, Chile, Brazil, and Peru [79–84].
In Brazil, only few cases of human trematodosis caused by heterophyids have been reported; these were from São Paulo [85–87]. However, records of metacercariae in mullets from off the Brazilian coast are common [24,26,75,76,88–90]. Following a report of the occurrence of metacercariae of A. longa with a prevalence of 100% in mullets from the Rodrigo de Freitas Lagoon, Rio de Janeiro, an alert was given concerning the possibility of a related human disease [24,26]. The spleen was reported to be the most parasitized organ in these fishes, with 100% of prevalence, followed by the heart, intestinal wall, liver and muscles, with prevalence decreasing from 98 to 87%. In other organs, such as the stomach wall, brain, gonads and gall bladder, the prevalence decreased from 70 to 30% [26]. Associated experimental infections using only small pieces of muscle tissue to fed hamsters resulted in a higher intensity of infection during spring/summer than in autumn/winter. However, the potential risk of infection was considered to be high in view of the high prevalence and intensity of A. longa in the muscles of mullets throughout the year [26]. Gueretz et al. [91] reported the prevalence of A. longa of 87% in Mugil curema and 100% in Mugil liza off Santa Catarina, southeastern Brazil, confirming the high prevalence on mugilid fish. Similar prevalence indices have been found in mugilids from Colombia and Uruguay [18,73].
Although heterophyid species are already included in the List of Risk Classification of Biological Agents by the Brazilian Health Ministry [92], human cases are no longer reported. Studies on the viability of heterophyid metacercariae of A. longa have been perfomed [93,94] with methodological standardization performed by Borges et al. [95]. Metacercariae isolated from M. liza and incubated in mullet muscle tissue at different temperatures showed that all metacercariae were dead after heating for 15 min at 60˚C, 100˚C and 180˚C. When frozen, all metacercariae died after 2 hr of exposure to −35˚C and −20˚C, but 24 hr of exposure to −10˚C was necessary to kill all metacercariae in the fillets [95]. The specific procedures for inactivation of parasites was important to prevent outbreaks of trematodiases caused by A. longa. Regarding the species with zoonotic potential such as C. formosanus, H. pumilio and Cryptocotyle spp., human cases have not been so far reported.
CONCLUSIONS
Our current knowledge of heterophyid species occurring in SA has enabled us to have a comprehensive view of the species distribution, their hosts and studies on taxonomy and life cycles, along with their influence on their host and the environment in terms of behavioral studies and ecotoxicologic interactions with cyanobacteria.
Asian species of heterophyids are constantly under study in relation to human and animal infections, but, for SA species, these data are still scarce. In this region, information concerning the effects of these trematodes on host tissues is needed, as are new diagnostic approaches for a better understanding of the epidemiology of this trematodoses and its geographic distribution. Marked definida por portesclaudia
The identification of the emerging risk of heterophyid species as a human health hazard relating to the ingestion of raw fish represents a preventive instrument at the disposal of authorities, and molecular approaches under study will provide new insights for the development of diagnostic tools.
AVAILABILITY OF DATA AND MATERIALS
The DNA sequences generated as a part of this study were deposited to the GenBank (Table 2).
ACKNOWLEDGMENT
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (001), PAEF/FIOCRUZ and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Universal n. 449658/2014-7).
Notes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.