Seroprevalence of Tissue and Luminal Helminths among Patients in Hanoi Medical University Hospital, Vietnam, 2018
Article information
Abstract
A serological survey was performed using ELISA to estimate the prevalence of tissue and luminal helminthic infections among hospital patients in Hanoi region, Vietnam. An overall seroprevalence of tissue and luminal helminthiases was 64.0% (95% CI 61.2–66.8) among 1,120 patients who visited Hanoi Medical University Hospital, Vietnam in 2018. The highest seroprevalence was observed against Toxocara spp. (59.0%), followed by Strongyloides stercoralis (46.3%), Gnathostoma spp. (25.5%), cysticercus (12.8%), Angiostrongylus cantonensis (10.5%), Fasciola spp. (11.1%), and Clonorchis sinensis (8.7%). Mono-infection by one species (11.1%) was lower than multiple infections (53.0%) (P<0.05). The seroprevalence in males (59.3%) was lower than in females (66.2%) but not statistically significant (P>0.05). Children (<15 years) revealed lower seroprevalence (34.0%) than adults (68.4%), and the age group 51–70 years revealed the highest seroprevalence (76.0%). Among the seropositive patients, eosinophilia (≥8.0%) was noted in 80.2%. The present results suggested active transmission of various tissue and luminal helminths among people in Hanoi, Vietnam.
INTRODUCTION
Helminthiases are widely spread around the world, especially in tropical areas, where local people have poor hygiene and lower income economies, such as Africa and Asia, including Vietnam. For example, human fascioliasis has been reported in more than 70 countries in Europe, the Middle East, Latin America, the Caribbeans, Africa, Asia, and Oceania [1]. Clonorchiasis is distributed in many countries such as South Korea, China, Taiwan, Vietnam, Japan, and Russia with 35 million infected cases in the world, including 15 million in China [2]. Toxocariasis is widespread, including the Americas, Japan, Canada, Germany, England, Italy, France, and Russia; in some areas the infection rate is up to 35–42% [3]. Based on serological examinations, the prevalence of toxocariasis has been reported to be 30–93% in developing countries [3]. Angiostrongyliasis due to Angiostrongylus cantonensis is distributed in more than 30 countries in Asia, Africa, the Caribbeans, Australia, and USA (Hawaii and Louisiana) with over 2,800 cases [4]. Strongyloidiasis caused by Strongyloides stercoralis is also widespread in the world with 30–100 million cases and are the cause of diseases of the intestine, lungs, skin, and others [5]. Gnathostomiasis has been detected in many countries, including Japan, Thailand, Cambodia, Laos, Myanmar, Indonesia, the Philippines, Malaysia, Vietnam, China, India, and Sri Lanka [6,7]. Cysticercosis due to infection with the metacestode of Taenia solium is distributed in wide areas of Asia, Africa, the Americas, and Oceania. In some areas, the prevalence of cysticercosis was 10% where neurocysticercosis was the cause of severe cerebral diseases [8].
In Vietnam, helminthic diseases are distributed all over the country, including fascioliasis in 63 of 63 provinces, clonorchiasis/opisthorchiasis in 32 of 63 provinces, and cysticercosis in 50 of 63 provinces; toxocariasis, strongyloidiasis, angiostrongyliasis, and gnathostomiasis are detected in every province of the country [9–12]. However, there have been no recent data to estimate the present status of these helminthic infections among hospital patients around Hanoi region. Therefore, the present study was performed to estimate the prevalence of tissue and luminal helminthiases, including toxocariasis, strongyloidiasis, gnathostomiasis, cysticercosis, fascioliasis, angiostrongyliasis, and clonorchiasis, using ELISA among the patients who visited Hanoi Medical University Hospital during 2018 (from 27 of 28 northern provinces), Hanoi, Vietnam.
MATERIALS AND METHODS
Sample collection
A total of 1,120 patients from 5 to>70 years-old who visited Hanoi Medical University Hospital in 2018 for serological examinations for helminthiasis were subjected in this study. Hanoi Medical University Hospital receives patients from throughout the entire country; however, in this study only patients from northern Vietnam were considered for convenience. ELISA test for detection of antibodies against parasitic agents was applied as a routine diagnosis by the Parasitology Laboratory of the hospital. All patients agreed to this study, including adults and children from 5–16 year-olds who gave consent through their parents.
Data analyses
The reason why the patients came to the hospital was collected for this study, 1 main symptom per patient only. Blood samples of all patients were analyzed for helminthic infections with an IgG ELISA test kit (DRG Instruments GmbH, Springfield, Illinois, USA), including toxocariasis, strongyloidiasis, gnathostomiasis, cysticercosis, fascioliasis, angiostrongyliasis, and clonorchiasis. The sensitivity and specificity of ELISA for these helminthic diseases were both satisfactory (>95%; data not shown). The seroprevalence was analyzed by locality of origin (from each province), mono-/multi-infection, gender (male/female), age groups (5–15; 16–30; 31–50; 51–70; and>70 years-old), and eosinophilia (≥8%, according to the normal physiology of Vietnamese inhabitants).
RESULTS
Chief complaints of patients
The main reasons (i.e., chief complaint) why 1,120 patients came to the hospital was headache 26.6%, stomachache 23.7%, digestive disorders 16.0%, allergy 13.6%, fever 10.3%, cough 7.1%, and loss of body weight 2.9%.
Geographical distribution in northern Vietnam
Twenty seven of 28 northern provinces (Fig. 1) had patients who visited Hanoi Medical University Hospital for examination of tissue or luminal helminth infections. The largest number came from Hanoi (112 patients), followed by Thai Binh (101), while the lowest numbers from Dien Bien and Bac Kan Province (Table 1).

Map of Vietnam showing administrative divisions of provinces. (A) Location of Vietnam in Southeast Asia (source: google). (B) Map of 28 provinces in northern Vietnam (magnification of the boxed area in Fig. 1A) showing the number of patients who visited Hanoi Medical University Hospital by different colors (for province names see corresponding numbers in Table 1).
Prevalence of helminthiasis
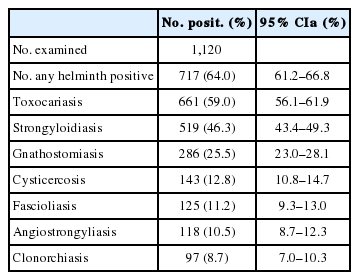
Of the 1,120 serum samples tested by ELISA, the overall helminthic infection rate was 64.0%, including Toxocara spp. infection 59.0%, Strongyloides stercoralis infection 46.3%, Gnathostoma spp. infection 25.5%, cysticercosis 12.8%, Fasciola spp. infection 11.2%, Angiostrongylus cantonensis infection 10.5%, and Clonorchis sinensis infection 8.7% (Table 2).
Mono-infection and multiple infections
The proportion of patients with mono-infection (11.1%) was lower than that with multiple infections (53.0%) (P<0.05). Toxocara mono-infection was 3.1% (multiple infections with Toxocara spp. and other helminths, 55.9%), and Strongyloides mono-infection was 2.3% (multiple infections, 44.0%). Mono-infection of Fasciola was 2.1% (multiple infections, 23.4%), and those of Angiostrongylus and Gnathostoma were 1.3% (multiple infections, 11.4%) and 0.98% (multiple infections, 10.2%), respectively. Cysticercus mono-infection was 0.71% (multiple infections, 9.8%), and C. sinensis mono-infection was 0.45% (multiple infections, 8.2%).
Helminthic infections according to gender
The seroprevalence of helminths in males (59.3%) was lower than that in females (66.2%) but without statistical significance (Table 3). Toxocara infection in males (55.9%) was lower than that in females (60.4%), and Strongyloides infection in males (43.8%) was slightly lower than that in females (47.5%). Gnathostoma infection was similar in males (25.7%) and females (25.5%), and cysticercosis was lower in males (14.4%) than in females (12.0%). Fasciola infection was slightly lower in males (10.7%) than in females (11.4%), and Angiostrongylus infection was lower in males (9.4%) than in females (11.2%) but without statistical significance in both infections. The gender difference in the seroprevalence was statistically significant only in Clonorchis infection; its prevalence was significantly higher in males (12.1%) than in females (6.8%) (Table 3).
Helminthic infections according to age group
The prevalence of helminthic infections was the highest in 51–70 years (76.0%) followed by 31–50 years (72.4%) and the lowest in 5–15 years group (34.0%) (Table 4). With the exception of angiostrongyliasis and clonorchiasis, the seroprevalence was the highest in 31–70 years of age (72.4–76.0%) compared with other age groups (Table 4). In angiostrongyliasis, 5–15 years group showed the highest seroprevalence (11.8%), and in clonorchiasis, the highest prevalence was observed in>70 years group (14.8%) (Table 4).
Overall, the seroprevalence of helminthiasis was lower in children (34.0%) than in adults (68.4%) with positive statistical significance. Toxocara infection in children (29.9%) was significantly lower than that of adults (63.3%), and Strongyloides infection in children (17.4%) was markedly lower than that of adults (50.6%). Gnathostoma infection in children (10.4%) was significantly lower than that of adults (27.8%), and cysticercosis in children (4.9%) was significantly lower than that of adults (13.9%). Fasciola infection in children (4.9%) was also significantly lower than in adults (12.1%), and clonorchiasis in children (1.4%) was markedly lower than in adults (9.7%). No age tendency was observed in the seroprevalence of Angiostrongylus infection in children (11.8%) vs adults (10.4%).
Eosinophilia by helminthic infections
Most (80.2%) of the helminth-infected patients had eosinophilia (≥8.0%), and a few (18.8%) seropositive patients revealed normal eosinophil (<8.0%) levels.
Helminthiasis according to the time of months in the year
The patients who visited our hospital for diagnosis and treatment of helminthiases came during every month of the year but the number of patients was different by month. The number of patients increased from January to the highest in June, and decreased thereafter to December (data not shown).
Anthelmintic treatment of seropositive patients
The seropositive patients were treated with anthelmintic drugs. In toxocariasis and gnathostomiasis patients, albendazole 15 mg/kg body weight/day×21 days was given; strongyloidiasis patients were given ivermectin 0.2 mg/kg/day for 2 days; angiostrongyliasis patients were prescribed albendazole 15 mg/kg×15 days; cysticercosis patients was treated with praziquantel 15 mg/kg in a single dose in the first day and then albendazole 7.5 mg/kg×2 times a day×20 days; fascioliasis patients were given triclabendazole 10 mg/kg×2 times a day for 1 day; clonorchiasis patients were treated with praziquantel 25 mg/kg×3 times a day for 2 days.
DISCUSSION
The most common reasons (clinical complaints) for the patients who came to the hospital included headache (26.6%) and stomachache (23.7%). Of the 1,120 patients who were from 27 of 28 northern provinces in Vietnam and came to Hanoi University Hospital for examination, 64.0% (717 patients) showed seropositive results for tissue or luminal helminthiasis. The number of patients from Hanoi City was the highest (112 patients) but the helminthic infection rate was the lowest (59.8%); the highest infection rate was observed from Lai Chau Province (75.0%).
Toxocara spp. infection rate was 59.0%, which was the highest in this study compared to previous reports [11–15]. In the laboratory of hospitals and medical centers, Toxocara infection was 45.2% [12] and 65.1% [13] before this report. In the community, Toxocara infection was 24.4% in southern provinces [14] and 17% in middle provinces [15]. The seroprevalencse of toxocariasis was reported from other countries; in Nigeria 30%, Brazil 36%, Swaziland 44.6%, Malaysia 58%, Indonesia 63.2%, Nepal 81%, Marshall Islands 86.8%, and La Reunion 93% [3,16]. The prevalence of Toxocara in rural areas was higher than that in urban areas (35–42% and 2–5%, respectively) [3]. The remarkably high seroprevalence of toxocariasis in northern Vietnam is probably due to high soil contamination with canine (Toxocara canis) or feline (Toxocara cati) feces containing the infective eggs.
The seroprevalence of S. stercoralis infection in this study was 46.3%. In other studies from Hanoi Hospital, the prevalence was was 20.0% [17], and in Ho Chi Minh Hospital 29.0% among the stomachache patients group [11]. In the laboratories, the prevalence was 22.5% [13] and 7.4% [12], and in the community 7.6% [18] and 17.3% [14]. S. stercoralis prevalence in other countries was 17.5% in Cambodia, 26.2% in Laos, and 23.7% in Thailand [19].
The seroprevalence of Gnathostoma spp. (mostly presumed to be G. spinigerum) infection in our study was 25.5%. In the Ho Chi Minh Hospital, Gnathostoma infection was 15.6% [13], but in the community, the prevalence was 6.8% in the middle of the country [20] and 17.3% in the south of the country [14]. The prevalence of cysticercosis in this study was 12.8%. In Ho Chi Minh Hospital, the prevalence was 9.8% [13] and 4.9% [12]. In the laboratory of Hanoi Hospital, the prevalence was 15.8% [9], and in the community, the infection rate in the north was 7.2%, and that in the south was 4.3% [9] and 5.1% [14].
Fasciola spp. infection was positive in 11.2% among 1,120 examined patients. In the laboratory of Ho Chi Minh Hospital, its prevalence was 11.2% [13] and 5.9% [12]. In the community, Fasciola infection rate was 11.0% [21] and 7.8% [22] in the middle of the country, and that in the north was 19.3% [14]. A. cantonensis infection was positive in 10.5%. In another study using PCR technique demonstrated that A. cantonensis was responsible for 67.3% of 55 cases of eosinophilic meningitis from a cohort of 1,690 adult patients at a tertiary hospital in southern Vietnam [23]. The prevalence of C. sinensis infection was 8.7%. In the community, where local people used to eat raw fish, the prevalence was up to 40.0% [9]. C. sinensis infection rate was higher in males than in females.
Most of the patients in this study had multiple infections (53.0%) and mono-infection was lower (11.1%); most of the patients were infected with 2–3 helminth species. The highest prevalence was seen in the age group of 51–70 (76.0%), and the lowest was in the age group of 5–10 (34.0%). In another study in Ho Chi Minh Hospital, the proportion of multiple helminthiases was 45.7–54.3% [12]. In the community, it was shown that Toxocara infection in the age group of >15 years was higher than that in the age group of ≤15 years (19.9% and 12.8%, respectively) [15]. The number of patients was the highest in May, June, and July, and this was similar with the results of De et al. [10] for a fascioliasis study in 2020 [10].
In conclusion, the overall seroprevalence of helminthic infections in the Parasitology Laboratory of Hanoi Medical University Hospital in 2018 was 64.0%, which included toxocariasis (59.0%), strongyloidiasis (46.3%), gnathostomiasis (25.5%), cysticercosis (12.8%), fascioliasis (11.2%), angiostrongyliasis (10.5%), and clonorchiasis (8.7%). Most of the patients had multiple infections with 2–3 species of helminths. Eosinophilia was observed in 80.2% of the total seropositive patients.
ACKNOWLEDGMENTS
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under a grant no. 106-YS.05–2014.08, and collaborated with the National Hospital of Pediatrics and Bach Mai Hospital, Hanoi, Vietnam.
Notes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.