Abstract
The third-stage larvae (L3) of the parasitic nematode, Anisakis simplex, have been implicated in the induction of hyperimmune allergic reactions in orally infected humans. In this work, we have conducted a review of an investigation into immune reactions occurring in animals experimentally infected with A. simplex L3. The patterns of serum antibody productions in the experimental animals against excretory-secretory products (ESP) of A. simplex L3 contributed to our current knowledge regarding specific humoral immune reactions in humans. In our review, we were able to determine that L3 infection of experimental animals may constitute a good model system for further exploration of immune mechanisms and allergy in anisakiasis of humans.
-
Key words: Anisakis simplex, immune reaction, IgE, allergy, ELISA, rat
INTRODUCTION
The whale nematode,
Anisakis simplex, a member of the superfamily Ascaridoidea, has been shown to induce stomach pain or allergies in humans. The adults of this parasite resides in the stomach of marine mammals, including fur seals, sea lions, and whales. In contrast to other species in this superfamily (e.g.
Ascaris lumbricoides in humans,
Toxocara canis in dogs, and
Ascaris suum in pigs) that do not require an intermediate host,
A. simplex must employ marine fish or cephalopods as second intermediate hosts for completion of its life cycle. Humans become infected with
A. simplex (hereafter referred to as anisakiasis) via the consumption of an intermediate host contaminated by its third-stage larvae (L3). After ingested orally, L3 penetrate into the gastric or intestinal wall, thereby inducing severe pain and strong immune responses in humans. The pain induced by L3 disappears after endoscopic removal of the worm. Some of the penetrating L3 can invade the peritoneum and eventually the larvae die with formation of parasitic granulomas surrounded by eosinophils and fibroblasts (
Sakanari et al., 1988;
Jones et al., 1990;
Daschner et al., 2000). The attendant host immune reactions elicited by the oral infection with L3 necessitated an intensive investigation in order to gain greater insight into the allergy associated with L3. This review aimed to focus on the immunology of anisakiasis studied in various experimental animals.
L3 infection induces the production of specific antibodies and cytokines (
Kennedy et al., 1988;
Daschner et al., 2001;
Nieuwenhuizen et al., 2006). Antibodies can be detected 2 weeks after infection, consistent with the time courses associated with other microorganisms. Analyses of specific antibody levels are generally irrelevant to the differential diagnosis of an acute state in cases of L3 infection, because the profound pain associated with L3 penetration begins only a few hours after the consumption of infected raw fish. However, antibody level measurements are helpful both in the differentiation of tumors from the granulomas formed by infiltrating L3 and in investigations of allergic diseases (
Gutierrez and Cuellar, 2002;
Kim et al., 2006).
The production of IgE tends to increase during parasite infections, but the ultimate effects of IgE vary considerably, depending on the host-parasite relationships. Hyperimmune allergic reactions have been closely associated with IgE production. The infection of a parasite into its normal host tends to reduce the development of allergic responses, despite the associated upshift in IgE production (
van den Biggelaar et al., 2000;
Yazdanbakhsh et al., 2002). By contrast, the infection of
T. canis in humans, an abnormal host, induces increased allergic reactions (
Sharp and Olson, 1962;
Sharghi et al., 2001).
A. simplex L3 has also been shown to induce allergic diseases at a high rate, principally due to the fact that humans are not a regular host of this parasite (
Audicana et al., 2002;
Klimpel et al., 2004).
Through investigations of allergic responses to L3 for a period of more than 10 years, several shared features have been identified, which indicate that immune reactions to L3 infection evidence similar patterns in humans and experimental animals (
Audicana et al., 2002). Although this remains a matter of some controversy, infections with living, and not dead L3 appears to elicit allergic reactions (
del Pozo et al., 1997;
Daschner et al., 2000;
Audicana et al., 2002;
Alonso-Gomez et al., 2004). After the report by Kasuya that allergies induced by the mackerel were actually the result of L3 contamination in the mackerel but not the mackerel itself, many researchers have detected species-specific antigens for the diagnosis of L3-dependent allergies (
Yakunin and Hallenbeck, 1998;
Asturias et al., 2000;
Perez-Perez et al., 2000;
Caballero and Moneo, 2002;
Shimakura et al., 2004). These inquiries improved L3-dependent allergy diagnoses, and provided a simple method for the resolution of the cross-reactivity problem (
Pascual et al., 1997;
Fernandez-Caldas et al., 1998;
Guilloux et al., 1998;
Cho and Cho, 2000;
Johansson et al., 2001). A thorough understanding of L3-dependent allergies requires the use of a variety of tissue preparations and in vivo reactions. However, researches into human immune reactions to L3 have been generally restricted to in vitro analyses (
Daschner and Pascual, 2005;
Del Rey Moreno et al., 2006).
Since the morbidity of allergy has increased, intensive investigations have been conducted regarding the mechanisms underlying L3-dependent allergies (
Isaac-Sterring-Committee, 1998;
Bochner and Busse, 2005). In particular, experimental animal models allowed investigations of in vivo reactions, as well as the observation of rapidly changing immune responses over time, both of which contribute to our current knowledge of the disease progress, which is required in order that better therapies can be developed. Investigations into immune reactions and allergic responses to larvae using experimental animals should contribute to the development of treatments for both allergies and parasitic infections.
EXPERIMENTAL ANIMALS USED FOR L3 INFECTIONS
The variables to be analyzed and their applications in human immunology differ according to the type of experimental animal employed. Syntheses of results from several animal models (rabbits, mice, and rats) have yielded the greatest amount of information regarding human immune reactions against L3. Rabbits weighing approximately 2 kg can allow easy bleeding in relatively large volumes. In rabbits infected orally with L3, approximately half in number of the L3 were retained within the host peritoneum and intestinal wall on day 13 post-infection (PI), which is expected to provide L3 antigens in sufficient quantity to trigger antibody synthesis by the host (
Hong and Lee, 1987). Rabbits were used in a study concerning IgE, but no recent research has pursued any active application of rabbit IgE in an effort to elucidate the mechanism of allergy development (
Hogarth-Scott 1967). Responses in rabbits, which are herbivorous, may be quite different from allergic reactions seen in omnivorous humans.
Mice are animals most frequently employed in studies of immune reactions. Mice weigh only approximately 20 g, and are not sufficiently large for oral infection with L3, that are 30-40 mm long and 1-2 mm wide. Consequently, L3 have been inoculated directly into the mouse peritoneum for antigen sensitization (
Jones et al., 1990). This direct inoculation route is justified by the fact that L3, when utilized via the oral infection route, ultimately move to the peritoneum through penetration of the gastric wall, in which they induce a strong immune reaction, again principally in the peritoneum (
Amano et al., 1995;
Iglesias et al., 1995). Inoculation of the peritoneum with L3 in mice has been shown to induce vigorous cellular reactions and an evident immune response (
Jones et al., 1990). Antibodies to L3 excretory-secretory products (L3-ESP) and 4th stage larva (L4) ESP (L4-ESP) were generated, indicating that L3 inoculated into the peritoneum becomes L4, and that each larval stage generates specific ESP. Antibodies to each ESP were also identified in oral L3 infections (
Kagei and Isogaki 1992;
Iglesias et al., 1995;
Hwang et al., 2003).
Rats weigh approximately 200 g, and have also been employed extensively in investigations of immune reactions against anisakiasis. Rats are approximately 10 times larger than mice, allowing weekly blood harvests. In addition, rat IgE levels can be evaluated via a variety of methods, and biological effects of rat IgE have been fairly well described. In an early investigation of experimental anisakiasis, Amano et al. (
1995) reported that the majority of L3 in orally infected rats was lost from the body after 1 day, and Cho et al. (
2006) reported that no L3 was found in the rat gastrointestinal tract at 1 week PI. Based on the short retention of L3 by rats, oral infection has been predicted to elicit an irregular antibody production and inconsistent immune reactions. Also, early investigations of rat immune responses to L3 utilized peritoneal inoculation of L3 for antigen sensitization. The allergic reactions of rats have also been analyzed by passive cutaneous anaphylaxis (PCA), via the inoculation of rat serum. However, Cho et al. (
2006) recently indicated that the L3 in orally infected hosts exerted constant effects on rat immune reactions, with regard to certain parameters.
EXCRETORY-SECRETORY PRODUCTS OF A. SIMPLEX LARVAE
A. simplex L3 larvae, which reside in marine fish and marine cephalopods, transform to L4, when the worms infect marine or land mammals. Several investigations have demonstrated superiority of L3-ESP in terms of antigenicity over crude
Anisakis larvae protein extracts, and the majority of research in this field has involved the analysis of immune reactions using ESP. Investigations into ESP protein constituents have determined that the ESP components from each larval stage are unique. Larval ESP is prepared by concentration of larvae culture supernatants harvested from the laboratory larval cultures of
Anisakis. In the laboratory, L3 become L4 around culture days 3 or 4, whereas this transition occurs within a few hours after infection in humans. Coomassie blue staining of SDS-PAGE gels has revealed differences in protein constituent components on culture day 4 in ESP prepared via daily harvesting of the culture supernatants (
Iglesias et al., 1995;
Hwang et al., 2003). The ESP harvested up to culture day 3 has been designated L3 ESP, and ESP after 3 days has been designated L4 ESP.
The diversity of the human immune responses to
Anisakis larval infections was previously evaluated by Hwang et al. (
2003), who demonstrated that each person generated specific antibodies, with differing ratios of L3-ESP to L4-ESP. On the basis of the results of indirect enzyme-linked immunosorbent assay (ELISA), human antibody production has been categorized into 4 reaction types; high specific antibodies to both ESPs, high specific antibodies either to L3-ESP or L4-ESP, low specific antibodies to the other, and low specific antibodies to both ESPs. In Western blots using L4-ESP as the antigen, the visualized antigen bands were relatively correlated with the levels of specific antibodies, identified by indirect ELISA in terms both of intensity and number. By contrast, L3-ESP antigen bands recognized by sera, in which high antibody levels were detected, did not differ significantly from the bands of sera, in which low levels of antibodies were detected, on the basis of the reactivity to L3-ESP via indirect ELISA. These differences between the results of indirect ELISA and Western blotting resulted in the profiling of the protein compositions of each ESP, using Microcon YM-100 and YM-3 filters (Millipore, Billerica, Massachusetts, USA). The majority of L3-ESP consists of low molecular weight (MW) proteins or peptides, and the serum antibodies from the rat anisakiasis serum were not found to bind to any of the low MW proteins (
Kim et al., 2005). L3-ESP harbors a low proportion of proteins in excess of YM-100 kDa, and a high proportion of those less than YM-3 kDa (for simplicity, the material recovered from the YM-100 filters was described as being in excess of YM-100 kDa, and the material filtered through YM-3 filters was described as less than YM-3 kDa). L4-ESP harbors a high proportion of proteins in excess of YM-100 kDa and a low proportion of those less than YM-3 kDa. The contrasting protein composition between L3-ESP and L4-ESP is an interesting phenomenon, which calls for further examination.
The secretion of proteinase by
A. simplex larvae has been reported previously by several investigators (
Sakanari and McKerrow, 1990;
Hotez et al., 1994;
Morris and Sakanari, 1994). In particular, neutral protease activity was detected in larval culture supernatants harvested from 24 hr cultures, which suggests that L3-ESP harbors a considerable amount of neutral protease (
Sakanari et al., 1982;
Matthews, 1989). The larval culture medium was maintained at 37℃ with a pH of approximately 6.5, which would allow for high neutral proteinase activity. The neutral protease detected in L3-ESP may function in the splitting of L3-ESP into smaller fragments. Mammalian stomachs normally harbor highly acidic gastric juice (pH 2.0), which would result in a reduction in neutral protease activity. Accordingly, the L3-ESP produced by L3 in the stomach would not be fragmented as an effect of neutral proteinase. The non-fragmented protein from L3-ESP has been regarded as sufficiently large to be processed by antigen-presenting cells and presented to T cells, resulting in activation of T cells. This T cell activation would result in an increase in the production of several rat antibody isotypes specific for L3-ESP, including IgE (
Kim et al., 2005).
In humans anisakiasis, sera can evidence relatively high levels of antibodies to L3-ESP, thereby indicating that the low MW proteins present in higher proportions in L3-ESP may evidence some antigenicity. The antigenicity of these low MW proteins is a subject that is worthy of further investigation. The synthesis of diverse antibodies in cases of human anisakiasis may be ascribed to the state or condition of both the host and infecting larvae (
Hwang et al., 2003). The possible relationship existing between human leukocyte antigen (HLA) expression and anisakiasis antibody production types also invites exploration. Investigators should bear in mind that the constituents of culture medium can influence the antigens produced by the larvae. Thus, antigens prepared by laboratory culturing may be different from those generated within the gastrointestinal tract.
Overall, specific antibodies for L3-ESP using the immunochemical method were reproducibly present at lower levels, and were more difficult to detect than antibodies for L4-ESP in all analyzed rat sera. The antigenic inferiority of L3-ESP relative to L4-ESP was also observed in the immunochemical analysis of specific IgE. However, when biological allergenicity was assayed via activation of RBL-2H3 cells, L3-ESP was found to be comparable to L4-ESP. Biological allergenicity can be confirmed by observing binding of specific IgE to the allergen (
Kim et al., 2005). These conflicting results indicate that IgE generated in a biological allergic state may differ from the IgE identified by immunochemical methods, at least in the case of L3-ESP.
SPECIFIC IMMUNOGLOBULIN M PRODUCTION
Daschner et al. (
2002) suggested that allergy to L3 was established in persons re-sensitized to L3, and that the exceptional elevation of specific IgM production was the result of an antigen or epitope that was not recognized in the primary infection. In studies of human allergies, serum is usually not harvested after the primary infection, and serum collected after a secondary infection cannot be compared to that resulting from the primary infection, which makes it difficult to understand the development of allergies.
The kinetics of specific antibody synthesis has been assessed in cases of oral rabbit infections and peritoneal mouse infections. Specific rabbit IgM reached a peak 2 weeks after a primary infection, and then decreased (
Quan et al., 1991). By way of contrast, the specific mouse IgM was elevated immediately after L3 infection, and continuously increased for 8 weeks (
Iglesias et al., 1993).
In sera obtained from rats infected via peritoneal L3 inoculation, the levels of IgM specific for the L3 product were correlated with the numbers of infecting L3 (
Amano et al., 1995). L3 product-specific IgM levels were elevated quickly after a primary infection, and then slowly from 4 weeks to 8 weeks PI. This IgM synthesis pattern was detected in both rats and mice following peritoneal inoculations. Levels of specific IgM after reinfection were comparable to those detected following a primary infection.
IgM production kinetics differed in rats infected by oral inoculation rather than peritoneal inoculation. Cho et al. (
2006) detailed the time-course of specific antibody production in each isotype using L4-ESP, and rat sera prepared from rats orally infected twice with L3, with a 9-week interval between infections. In rats orally infected with 5 L3, specific IgM levels evidenced a peak 1 week after both primary and secondary infections. In rats orally infected with 20 L3, specific IgM peaked 2 weeks after both primary and secondary infections. In comparison to the high-level plateau induced by a peritoneal inoculation with L3, the level of specific IgM after an oral infection decreased continuously after its peak.
The rat sera prepared by repeated oral infections with L3 provided an opportunity to compare the immune reactions induced by primary and secondary infections, which are quite difficult to examine in humans. The time-course study showed that elevated specific IgM synthesis at 4 weeks PI (both primary and secondary) remained lower than peak levels (
Quan et al., 1991;
Yang et al., 1991;
Cho et al., 2006). If human-specific IgM synthesis is similar to that in rats, human IgM levels at 2 weeks after human allergy should be higher than at 4 weeks after human allergy. Antigen analysis by immunoblot showed that the specific rat IgM, which were elevated after reinfection, was not directed against antigens, unlike those observed after a primary infection (
Cho et al., 2006). Specific IgM production is not generally thought to rely on the memory of antigen sensitization, and thus specific IgM avidity should not increase following a secondary immunization. In cases of anisakiasis in rats, however, the specific IgM avidity in the secondary infection was higher than the avidity in the primary infection, which suggests that the characteristic elevation of specific IgM in human allergy, associated with L3 infection, may not be resultant from a new epitope not identified upon the primary infection (
Cho et al., 2006). No previous reports comparing IgM avidity between a primary immunization and a secondary sensitization were available. Infection with
A. simplex, which is parasitic in marine mammals, may cause unique and exceptional immune reactions in land-dwelling mammals.
SPECIFIC IMMUNOGLOBULIN G PRODUCTION
The initial investigation of human immune responses to L3 was conducted via the identification of specific IgG production (
Lee et al., 1990;
Yagihashi et al., 1990;
Daschner et al., 1998;
Lorenzo et al., 2000). Specific IgG levels were maintained at a high plateau level for a long time, which effectively simplified their detection and identification. At the same time, the measurement of specific IgG was not appropriate to differentiate the current infection from a previous exposure history. The kinetics of specific IgG production against L3 was determined in human anisakiasis allergy cases by Daschner et al. (
2002). Although somewhat controversial, specific IgG
4 production has been evaluated by investigators of allergic diseases, as IgG
4 binds to epitopes recognized by specific IgE, and is regarded as an indicator of allergic disease states (
Hagan et al., 1991;
Lambin et al., 1993;
Atmadja et al., 1995;
Garraud et al., 1995). In patients allergic to L3, the IgG specific for L4-ESP, once produced, was maintained at a high level for 6 mo. However, the IgG
4 specific for L4-ESP decreased more quickly than did overall IgG. In investigations of any relationship between parameters via the analysis of correlations, no noticeable findings were described (
Daschner et al., 2002). To determine any such relationship, measurements of more than 3 time points were required.
In orally infected rabbits, the production of IgG specific to L3 protein extract achieved a peak at 30 days post primary infection (about 4 weeks), and then decreased (
Hong and Lee, 1987). Yang et al. (
1991) reported that rabbit IgG achieved a high level plateau between 3 and 7 weeks post primary infection, and then decreased. Quan et al. (
1991) also demonstrated peak rabbit IgG production against
A. simplex larval ESP or crude L3 extract at approximately 4 to 5 weeks after a primary infection.
The IgG rodent subtype differs from that of humans. Rodent IgG does not include any IgG subtype equivalent to human IgG
4, the IgG subtype which shares the epitope for the IgE binding site. Instead, rodent IgG production is controlled by a different cytokine. The cytokines inherent to the control of rodent IgG production differ depending on the IgG subtype. In mice, subtypes IgG
1, IgG
2b, and IgG
3 are affected by cytokines secreted by the Th2-type T-helper lymphocyte subset, whereas IgG
2a is stimulated by the Th1 cytokine, IFN-γ (
Finkelman et al., 1988). In rats, the production of IgG
1 and IgG
2a is increased by the Th2 cytokine IL-4, and IgG
2b and IgG
2c are controlled by IFN-γ (
Binder et al., 1995;
Cetre et al., 1999). Analyses of IgG subtype production in rats seem to be useful in evaluation of the immune responses in humans (
Fig. 1).
Specific IgG production after peritoneal L3 inoculation in mice was shown to differ from the results observed in orally infected rabbits. In contrast to the reduction of specific IgG observed 5 weeks post primary infection in rabbits, the specific IgG1 in mice increased continuously for up to 8 weeks after the primary infection. We noted a steady increase in specific IgG2a production in mice following inoculation. As IgG2a synthesis in mice is controlled by IFN-γ, IFN-γ production is generally regarded to be increased after infection with L3.
The production of specific rat IgG after peritoneal inoculation steadily increased to a high level plateau, which did not decrease until 2 mo post inoculation (
Amano et al., 1995). Such maintenance of a high level response can also be observed in mice after a peritoneal infection. Similar maximum levels of IgG were observed in rats inoculated twice with 1 larva or 20 larvae. Cho et al. (
2006) reported that specific IgG
1 and IgG
2a levels were maintained at a high level plateau for more than 2 mo, whereas specific IgM levels peaked. This increase after antigen re-sensitization was faster and higher than after the primary infection, which indicates the role of memory cells. The timecourse of specific rat IgG production after an oral infection differed from the pattern of a peritoneal infection, with a small peak observed at the time of reinfection. However, this small peak was not regarded to be of any significance, as a relatively high level was maintained for a long period, of approximately 2 mo. The rats generated several IgG subtypes. Levels of IgG
1 and IgG
2a were strongly correlated over 16 weeks, which suggests that the same or some related mechanism controls the generation of IgG
2a and IgG
1. By contrast, the majority of rats orally infected with L3 did not generate IgG
2b, which is modulated by IFN-γ. Collectively, these results show that the oral infection of rats with L3 is mediated by a Th2 response, as opposed to the Th1-driven response observed following a peritoneal inoculation in mice.
SPECIFIC IMMUNOGLOBULIN A PRODUCTION
IgA antibodies are secreted through the mucosal layer lining the gastrointestinal tract, in order to prevent parasites from colonizing the intestinal tract.
A. simplex inhabits the gastrointestinal canals of marine mammals, and thus the production of IgA specific to L3 is generally regarded as a good marker for the immune response of land mammals to L3 infection. The synthesis of specific IgA in human anisakiasis has been reported (
Akao et al., 1990;
Lorenzo et al., 1999;
Daschner et al., 2002). Although some antigens have been determined not to induce specific IgA (
Lorenzo et al., 1999), the majority of humans infected by L3 evidenced specific IgA production.
Iglesias et al. (
1993) attempted to measure IgA specific for L3 in mice after peritoneal inoculation, but observed no meaningful or recognizable production. We have attempted to detect rat IgA specific for L4-ESP, but have detected no binding of specific IgA (unpublished data). Poor specific IgA production has also been detected in mice infected with
T. canis (
Cuellar et al., 2001). It may be that neither mice nor rats generate IgA specific to L3, which might prove an important difference between human and rodent immune responses to L3 infection.
SPECIFIC IMMUNOGLOBULIN E PRODUCTION AND ALLERGY TO L3
Allergies to L3 infection have previously been diagnosed by positive skin prick tests, specific IgE production, and total elevation of IgE levels (
Garcia et al., 1997;
Moreno-Ancillo et al., 1997;
Lopez-Serrano et al., 2000;
Moneo et al., 2000;
Purello-D'Ambrosio et al., 2000). Daschner et al. (
2002) has elucidated in detail the time course of IgE production in human L3-dependent allergies. On the basis of the results of immunochemical analyses, specific IgE levels were lowest at day 0 and highest 1 and 6 mo after the allergy episode, but few overall differences were observed among the time points. Total IgE levels at day 0, 1 mo, and 6 mo after exposure were found to be relatively similar. The use of an experimental animal model in the study of the kinetics of specific IgE and total IgE production provided useful data, which could not be readily obtained with humans.
Mice are a popular animal in laboratory studies concerning IgE (
Prouvost-Danon et al., 1967). No specific IgE was isolated after L3 inoculation into the peritoneum of mice (
Iglesias et al., 1993). Baeza et al. (
2005) successfully induced L3-specific IgE via the immunization of mice with L3 worm crude extracts, using pertussis toxin and alum as adjuvants (
Baeza et al., 2005). The intravenous injection of crude L3 extract into these immunized mice induced active cutaneous anaphylaxis and elevated serum histamine levels. However, the allergy model employing L3 crude extract requires some scrutiny. The analysis of splenocyte cytokine generation showed that, despite high levels of IL-10, the levels of IL-4 synthesis were relatively low, and IFN-γ production levels were extraordinarily high. In a typical immune reaction, the high IL-10 levels induce a high level of IL-4, and a reduction in the levels of IFN-γ. The response of the splenocytes observed in the allergy model is suggestive of blocked processing in the flow of the immune reaction. Splenocytes also did not contribute to the production of specific IgE, as the levels of IL-4, which increases IgE production, were low. This suggests that some other cell type may facilitate the production of specific IgE. Although the production of specific IgG
2a in mice was also observed after the inoculation of L3 into the peritoneum (
Iglesias et al., 1993), the production of specific IgG
2a induced by the injection of L3 crude extracts was greater than that seen with specific IgG
1, which indicates a Th1 response in the immune reaction greater than that associated with Th2. In addition, ESP tends to be preferred over crude larval extracts, as the detection of specific IgE against the ESP of
A. simplex larvae has proven superior to that observed against crude extracts, and a high level of specific antibody production against small volumes of ESP has been observed in other nematode infections (
Sugane and Oshima, 1983;
Sugane et al., 1985;
Cho, 2000).
Rats are also popular in investigations of the immune response, and are generally favored over mice in early allergy studies (
Ogilvie et al., 1966). PCA was frequently employed in order to identify biological allergenicity prior to the availability of a immunochemical IgE identification technique. Amano et al (
1995) insisted that the allergic reaction was more severe in cases of re-infection than in primary infection, which is consistent with the generally accepted allergy model. After the peritoneal inoculation of rats with L3, however, the levels of PCA reaction were related inversely to the numbers of infecting L3.
Both Kim et al. (
2005) and Cho et al. (
2006) utilized the oral L3 infection of rats to explore allergies to L3, and their findings have contributed to our understanding of allergies in humans. The identification of specific IgE is the initial step in the diagnosis of an allergic reaction. The specific production of IgE has normally been demonstrated via indirect ELISA, RAST, or Western blot, which is predicated on the principles of the immunochemical binding reaction. However, the levels of specific IgE measured via immunochemical methods have not been correlated with the severity of allergies, which has led to the suggestion that some specific IgE cannot be immunochemically detected (
van Ree and Aalberse, 1999;
Kontou-Fili, 2002).
Marie et al. (
1999) determined that the application of biological methods, including the skin prick test, contributed to the reduction of false positives for the detection of specific IgE. False positives in immunochemical analysis are predicated on cross-reactivity with carbohydrates attached to allergens. As a biological method, PCA may also reduce cross-reactivity, but PCA is available only in experimental animals, and not in humans. Investigations of rat IgE are relatively easy, as monoclonal antibodies to rat IgE are readily available, and the well-described RBL-2H3 mast cell line can be used to augment the biological activity of rat IgE (
Kulczycki and Metzger, 1974;
Conrad et al., 1983;
Dibbern et al., 2003). RBL-2H3 cells have features of rat mucosal mast cells, and have also been applied to the analysis of biological allergenicity or the allergic state, using rat serum. The results of RBL-2H3 activation have been highly correlated with that of PCA (
Siraganian et al., 1982;
Kawabata and Babcock, 1993;
Hoffmann et al., 1997). Currently, analyses of the biological allergic state of human sera can be conducted using RBL-2H3 that has been genetically manipulated to harbor human FcɛRI. The biological allergic activity of humans evidenced a weak correlation with the biochemical allergic levels (
Dibbern et al., 2003).
Cho et al. (
2006) assessed allergy progression via investigations of the timecourse of L3-dependent allergies in rats. Both immunochemically and biologically measured specific IgE levels were higher after reinfection than primary infection. However, total IgE levels, when immunochemically analyzed, did not differ between primary and secondary infection. The levels of total IgE were, however, affected by the numbers of L3 employed in the infection. Total IgE levels were highest after infection with 20 L3, lower with 5 L3, and even lower in the uninfected controls. Human total IgE production in allergy kinetics plateaued at a high level for 6 mo. The tendency towards extended high levels of rat IgE indicates that the rat model can be applied to human L3 allergy (
Daschner et al., 1999).
The highest peak of the immunochemical allergic state and the biological allergic state was observed at 2 weeks and 1 week after oral reinfection in rats, respectively (
Cho et al., 2006). Amano et al. (
1995) also observed the highest peak of the biological allergic state at 1 week post-reinfection.
Mitchelle et al. (
2002) evaluated the differences between the timing of maximum immunochemical and biological responses in greater detail, using chicken ovalbumin as an allergen and alum as adjuvant, and reported that the biological allergic state of rats was inversely correlated with specific rat IgE avidity. Cho et al. (
2006) did not observe the same relationship in response to L3-dependent allergies in rats that were orally infected with L3 in the absence of an adjuvant. A comparison between these findings may not be appropriate, as oral infection with L3 simulated a natural infection, and promoted responses to multiple antigens rather than a single protein, and the adjuvants were not applied uniformly. Indeed, these results appear to indicate that any investigation of factors that affect the biological allergic state should utilize L3 infected rats rather than artificial antigen injections, in order to simulate the natural processing of the L3 antigens more accurately.
Cho et al. (
2006) detailed the correlation between a variety of parameters against rat sera over the timecourse of the allergic reaction. The resultant graphs show the complexity of the situation, and provide a valuable basis for future investigations. Of particular interest, the correlation of specific IgM with other measured parameters changed dramatically at 1 week post-reinfection in comparison to neighboring time points. None of the available supporting data facilitated an explanation of this phenomenon. The poor correlation between the clinical severity of allergy and immunochemically identified specific IgE levels requires more intensive investigation (
Mari et al., 1999;
Epton et al., 2002;
Marchand et al., 2003).
As previously discussed, larval ESP preparation did not allow for the easy harvest of proteins in excess of 100 kDa, as L3-ESP harbored a proteinase with a high degree of activity. However, L3-ESP retained a high degree of biological allergenicity. A novel procedure for the preparation of L3-ESP including high MW proteins should be developed in the future. L3-ESP has proven to be a valuable tool in our understanding of the immune reaction to L3.
CONCLUSION AND PERSPECTIVES
In this work, we have reviewed the immunological characteristics of animals infected with A. simplex larvae. In order to determine the most appropriate animal model for study of human anisakiasis, laboratory investigations using in vivo systems continue to be conducted. Currently, immune reactions in rats are generally regarded to be similar with those of humans, particularly with regard to allergy progression. In order to explore and extend our current knowledge regarding allergy or immune reactions in abnormal hosts as the result of parasitic infection, anisakiasis in rats can be considered a good model for future investigations.
Notes
-
This work was supported both by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-015-E00099) and by the Brain Korea 21 Projects in 2006.
References
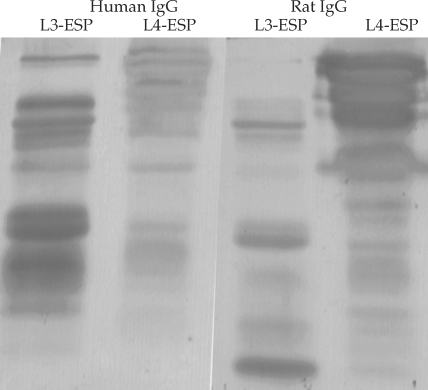
Fig. 11. Antigen bands of both L3-ESP and of L4-ESP localized with either human anisakiasis sera or rat sera infected with Anisakis simplex L3 larvae. Human sera (IgG) showed the antigen band visualized with stronger intensity at L3-ESP. The rat sera (IgG) showed the inversed state. However, most antigen bands were localized similarly between human sera and rat sera.