Evaluation of the anti-Toxoplasma gondii Activity of Hederagenin in vitro and in vivo
Article information
Abstract
Toxoplasma gondii infection is widespread worldwide, not only posing a serious threat to human food safety and animal husbandry, but also endangering human health. The selectivity index was employed to measure anti-T. gondii activity. Hederagenin (HE) exhibited potent anti-T. gondii activity and low cytotoxicity. For this reason, HE was selected for in vivo experiments. HE showed 64.8%±13.1% inhibition for peritoneal tachyzoites in mice, higher than spiramycin 56.8%±6.0%. Biochemical parameters such as alanine aminotransferase, aspartate aminotransferase, glutathione, and malondialdehyde, illustrated that HE was a good inhibitor of T. gondii in vivo. This compound was also effective in relieving T. gondii-induced liver damage. Collectively, it was demonstrated that HE had potential as an anti-T. gondii agent.
Toxoplasmosis is caused by T. gondii, which is a globally distributed parasite. This parasite infects many nucleated cells of mammals and humans and an estimated 2 billion people worldwide [1]. The disease is normally asymptomatic in hosts with normal immune function and no treatment is required. However, parasites can cause more serious and potentially life-threatening harm to people with immune deficiencies [2].
Clinically, a combination of sulfa drugs and dihydrofolate reductase inhibitors have been used to successfully treat and prevent various diseases caused by T. gondii [3]. This has played an important role in human anti-toxoplasmosis but these interventions can cause adverse reactions, including bone marrow suppression, hypersensitivity, hematologic toxicity and rashes [4]. These drawbacks highlight the need for drugs with anti-T. gondii activity that function with high efficiency, low toxicity and fewer side effects.
Natural products are considered promising reservoirs for drug discovery and are vital in drug development programs [5–7]. Hederagenin (HE) is a pentacyclic oleane-type triterpenoid found in large quantities in the pericarps of fruits of Sapindus saponaria (Sapindaceae) [8]. Several biological activities have been reported for HE, including antifungal [9], anti-leishmanial [10]. Due to such reports and our continued interest in the search for bioactive compounds from the natural products, we recently started to investigate the potential of HE as a source of new anti-T. gondii drugs. HE exhibited low cytotoxicity and high selectivity indices in in vitro studies and was selected for analysis in vivo. The research aimed to lay the foundation for elucidating mechanism study anti-T. gondii of compound HE.
Dulbecco’s Modifified Eagle Medium (DMEM), Penicillin-Streptomycin solution, Trypsin-EDTA, and Phosphate Buffered Saline (PBS) for cell culture were obtained from Biological Industries (Beijing, China). Fetal bovine serum (FBS) for cell culture was purchased from Gemini (California USA). Spiramycin (FW 843.05) was purchased from Aladdin Industrial Inc. (Shanghai, China). HE was obtained from Nanjing Spring& Autumn Biotech Co. Ltd (purity >98%). Each compound was dissolved in dimethyl sulfoxide (DMSO, Solarbio, Beijing, China) and diluted with DMEM to different concentrations, with the final DMSO concentration at less than 1% (v/v).
HeLa cells were cultured in DMEM, supplemented with 100 units/ml Penicillin and 100 μg/ml Streptomycin and 10% heat-inactivated FBS and maintained at 37°C and 5% CO2. Cells were purchased from American Type Culture Collection (Manassas, Virginia, USA). Tachyzoites used in our study were from the virulent RH strain of T. gondii and maintained by serial intraperitoneal passage in KM female mice. KM female mice were purchased from Experiment Center, Yanbian University. All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Yianbian University, Jilin, China (experiment alanimal ethics batch number IACUC-20170105039), and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Number of license SCXK 2011-0007). All mice were kept in a central animal care facility with free access to water and rodent food during the experiment.
HeLa cells were seeded onto the 96-well plates (3×103 cells per well) for 24 hr for obtaining the full monolayer, and then the host cells were infected with T. gondii (parasites: cells=5:1) in complete medium. After 24 hr, cells were washed to remove any extra parasites and then incubated with different concentrations (1–1,000 μM) of compounds served. Spiramycin was used as positive control. After 24 hr of incubation, 10 μl of 5 mg/ml MTT solution was added to each well. Plates were then incubated for a further 4 hr. IC50 in HeLa cells, IC50 in infected HeLa cells and selectivity index were calculated by Excel software. Selectivity index was a measure of specific resistance to Toxoplasma. Calculated using the formula shown in Table 1.
Female mice were randomly divided into 4 groups and then intraperitoneally injected with 2×103 tachyzoites of the T. gondii RH strain. After 4 hr of infection, mice were orally treated or not with different drugs: 0.2 ml normal saline (normal group and toxo control group), 100 mg/kg spiramycin (spi group), 100 mg/kg Hederagenin (HE group) according to the mice weight, respectively, once a day for 4 days. At the last day, eye blood samples were collected after anesthesia to separate the serum, and then mice were sacrificed by cervical dislocation. After that, the number of tachyzoites in the abdominal cavity of the mice was counted under the light microscope and the inhibition rate of the parasites was calculated. Then the liver and spleen were dissected. The determination of liver and spleen index, serum (Alanine aminotransfease) ALT, (Aspartate aminotransferase) AST, and liver homogenate (Glutathione) GSH and (Malondialdehyde) MDA were determined [13]. Determination of ALT and AST method: The substrate reaction of ALT or AST and serum was carried out under incubation at 37°C for 30 min, then added 2,4-dinitrophenylhydrazine (2,4-DNPH) and held for 20 min. Finally, NaOH was added and allowed to react for 5 min. The absorbance at 505 nm was measured. Determination of GSH method: The liver homogenate was mixed with half volume trichloroacetic acid (20%, w/v) and centrifuged at 4,000 rpm for 10 min. Then, phosphate buffer (phosphate 0.3 mol/L, pH 7.5) and 5,5-dithio-bis-(2nitrobenzoic acid) (0.04%, w/v) were added to the separated supernatant and mixed thoroughly. After 5 min at room temperature, the absorbance was measured at 412 nm. Determination of MDA method: The liver homogenate supernatant was mixed with thiobarbituric acid (0.5%, w/v) and heated in boiling water bath for 1 hr, then cooled quickly and centrifuged at 6,000 rpm for 10 min, the absorbance of pink colored supernatant was measured at 532 nm. Tetraethoxypropane replaced the liver homogenate in the standard sample. Another 18 mice were randomly divided into infected untreated group, infected with 100 mg/kg spiramycin-treated group (spi) and infected with HE-treated group, 6 in each group. Four hours after the infection, the mice were orally gavage as described above and administered once a day for several consecutive days to observe the survival rate of the mice. All data were expressed as mean±standard deviation of triplicate measurements.
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, California, USA). A value of P<0.05 was considered statistically significant.
In this study, HE was selected to evaluate their anti-T. gondii activity in vitro. When the SI level was higher than that obtained for spiramycin, the toxicity of the compound to the host cells (HeLa cells) was lower than spiramycin, and the compound reduced the T. gondii-induced infection of the host cells; then the compound was considered to have improved anti-T. gondii activity. The cell viability of T. gondii-infected HeLa cells was compared to uninfected HeLa cells and was expressed as a percentage change from the control value. The selectivity index (SI) is generally reflected the efficacy of a compound against T. gondii and toxicity for host cells, which was calculated using the formula: the ratio of the median toxicity concentration (CC50, IC50 in HeLa Cell) value for host cells not infected with T. gondii to the median inhibitory concentration (EC50, IC50 in T. gondii infected HeLa cells) for T. gondii cultivated in host cells (SI=CC50/EC50) [11].
The results are summarized in Table 1. HE exhibited stronger SI than the clinical toxoplasmosis medication spiramycin (SI=0.72). The compound HE contains 2 hydroxyl groups and one carboxyl group, and SI values greater than spiramycin. It is hypothesized that the hydroxyl groups interact with the receptor protein by hydrogen bonding thus resulting in their enhanced biological activity. HE had a high SI and also showed the lowest toxicity to HeLa cells. For this reason, HE was selected for further analysis in in vivo studies.
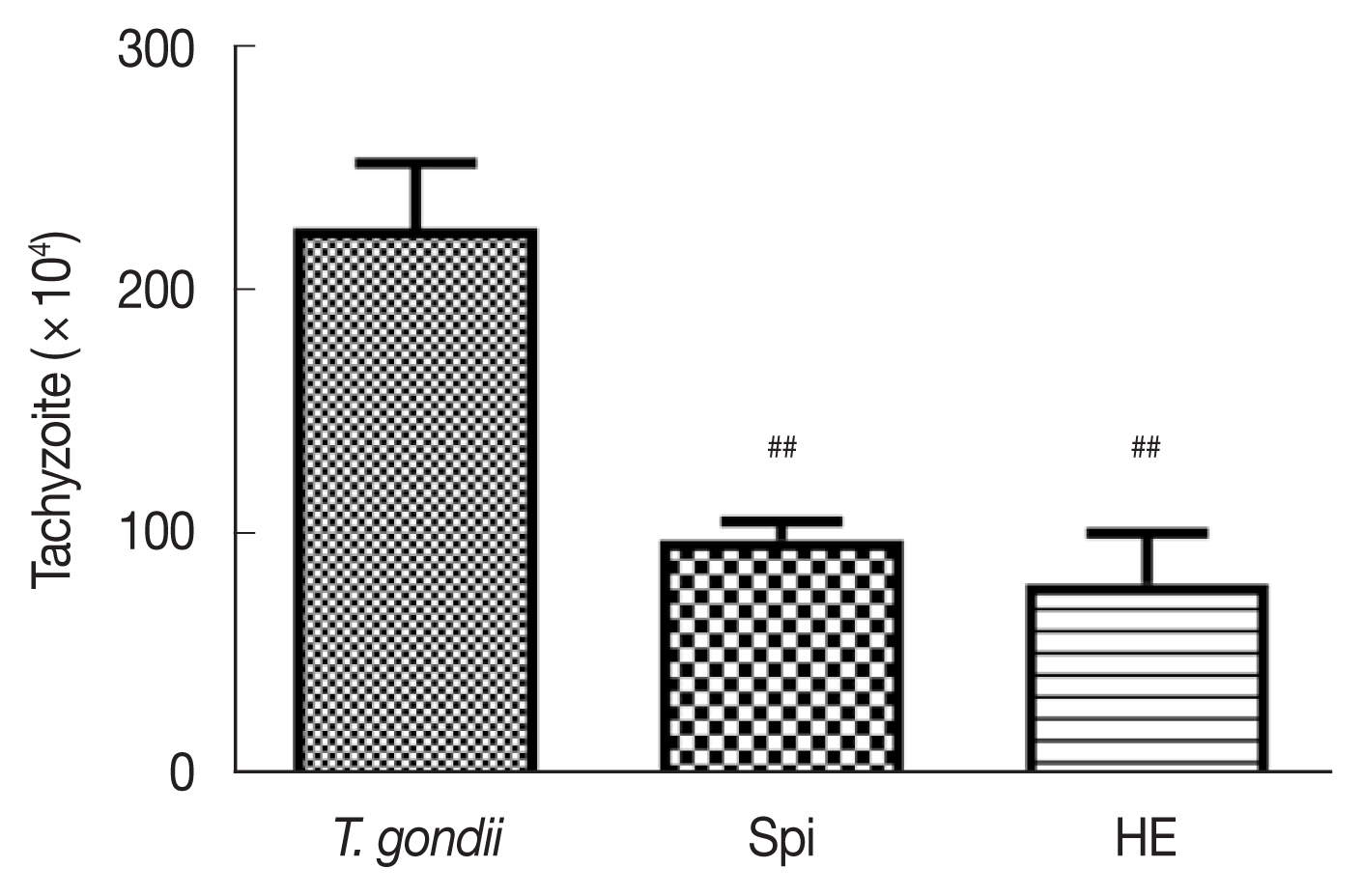
To determine whether HE exhibited anti-T. gondii activity in vivo, we infected female KM mice with a T. gondii RH strain to construct an acute T. gondii infection model. We administered HE and spiramycin by gavaging and then evaluated the number of T. gondii in the abdominal cavities of mice by counting [14]. The number of tachyzoites in the ascitic fluid of mice treated with 100 mg/kg spiramycin and 100 mg/kg HE decreased compared to the control mice; the inhibitory rates were 56.8%±6.0% and 64.8%±13.1%, respectively (Fig. 1). These results indicate that HE inhibited the activity of tachyzoites in vivo, and at a rate close to spiramycin.
Serum levels of ALT and AST activity are sensitive indicators of hepatotoxicity. Elevated levels of these enzymes provided indices of the degree of liver damage (Fig. 2A). Compared to the levels of ALT and AST in the control group, the serum levels of ALT and AST were increased in mice infected with T. gondii. This indicates that acute T. gondii infection could result in liver damage. The levels of ALT and AST were significantly reduced in the HE-treated mice when compared to the levels of the same enzymes in T. gondii infected mice and the infected mice that had been treated with spiramycin (P<0.05). These results indicate that HE could reduce the hepatotoxicity while targeting T. gondii.
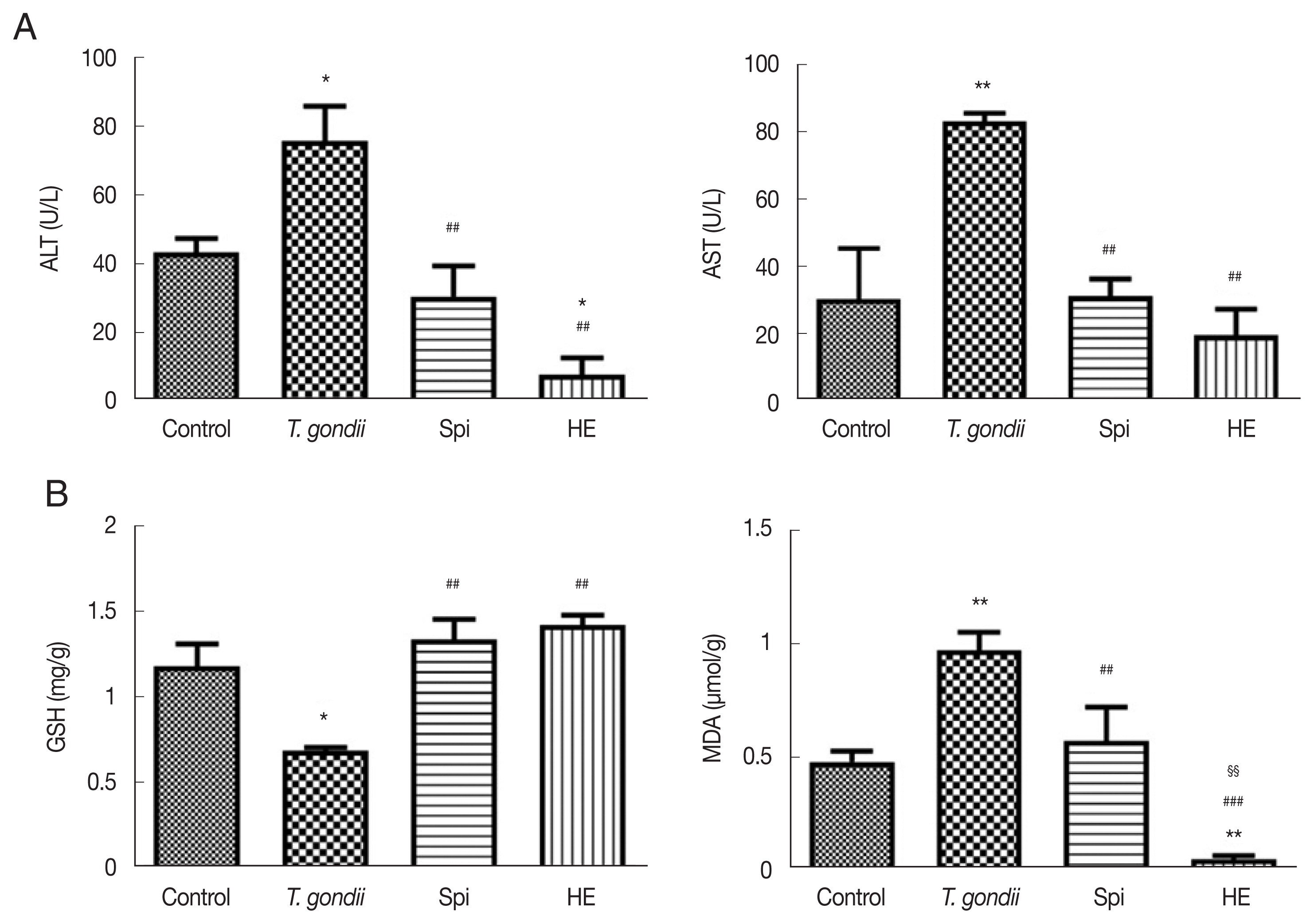
(A) Effect of compounds on ALT and AST levels in T. gondii-infected KM mice, n=6. (B) Effect of compounds on GSH and MDA levels in T. gondii-infected KM mice, n=6. *P<0.05 compared with the normal group; **P<0.01 compared with the normal group; ##P<0.01 compared with toxo group; ###P<0.01 compared with toxo group; §§P<0.01 compared with Spi group.
MDA is the major degradation product of lipid peroxidation and can cause changes in the structure of hepatocytes, leading to their swelling and necrosis [18]. The level of MDA reflects the degree of damage to the liver cells from an alternative perspective [19]. GSH, the major non-protein thiol in humans and other mammals, can react with peroxides to exert anti-oxidative effects and to reduce liver damage [20]. Compared to the control group, the glutathione (GSH) content in the liver homogenate of the T. gondii infected mice was significantly reduced (P<0.01). This indicated that an acute T. gondii infection caused a decrease in the anti-oxidant GSH, in mice (Fig. 2B). T. gondii infected mice that were treated with HE and spiramycin showed significantly elevated levels of GSH relative to the levels detected in the T. gondii infected mice.
The T. gondii infected mice exhibited higher levels of MDA compared to the control mice (P<0.01). Treatment with spiramycin and HE resulted in a reduction in the levels of MDA. After treated with HE, there was a small amount of MDA when compared to the control group (P<0.01). HE treatment resulted is a greater reduction of MDA than spiramycin treatment (P<0.01). Studies have shown that a Toxoplasma infection can cause a reduction in the body’s defense capacity. MDA may be elevated in the body to protect tissues from free radical damage [21]. The mechanism by which HE, an anti-oxidant, protects against a T. gondii infection may involve its anti-oxidative activity. This suggests that anti-oxidants may have potential in a therapeutic regimen for the treatment of T. gondii-related diseases [22].
After the administration of the infected mice, the length of their survival time can reflect the strength of the protective effect of the drug on the acutely infected mice with T. gondii. The longer the infected mice survive, the stronger the protective effect of the drug on the acutely infected mice. It can be seen from Fig. 3 that the mice in the toxo group and the HE group began to die on the 9th day, and the survival rate was 67%. On the 10th day, the mice in the Spi group began to die with a survival rate of 50%, while the survival rate of the mice in the toxo group and the HE group was 17%; On the 11th day, all the mice in the toxo group died, while the survival rate of the mice in the HE group and the Spi group was 17%. All mice died on the 12th day. These results show that both Spi and HE can control the pathological changes in the acutely infected mice to a certain extent, prolong the survival time of the mice, and have a certain protective effect on the acutely infected mice. From in vivo experimental data, HE did not show too ideal anti-T. gondii activity. The structure modification method can be used to make it an ideal anti-T. gondii drug. At the same time, a combination of drugs can be used to enhance the anti-T. gondii activity of HE and reduce some side effects caused by HE.
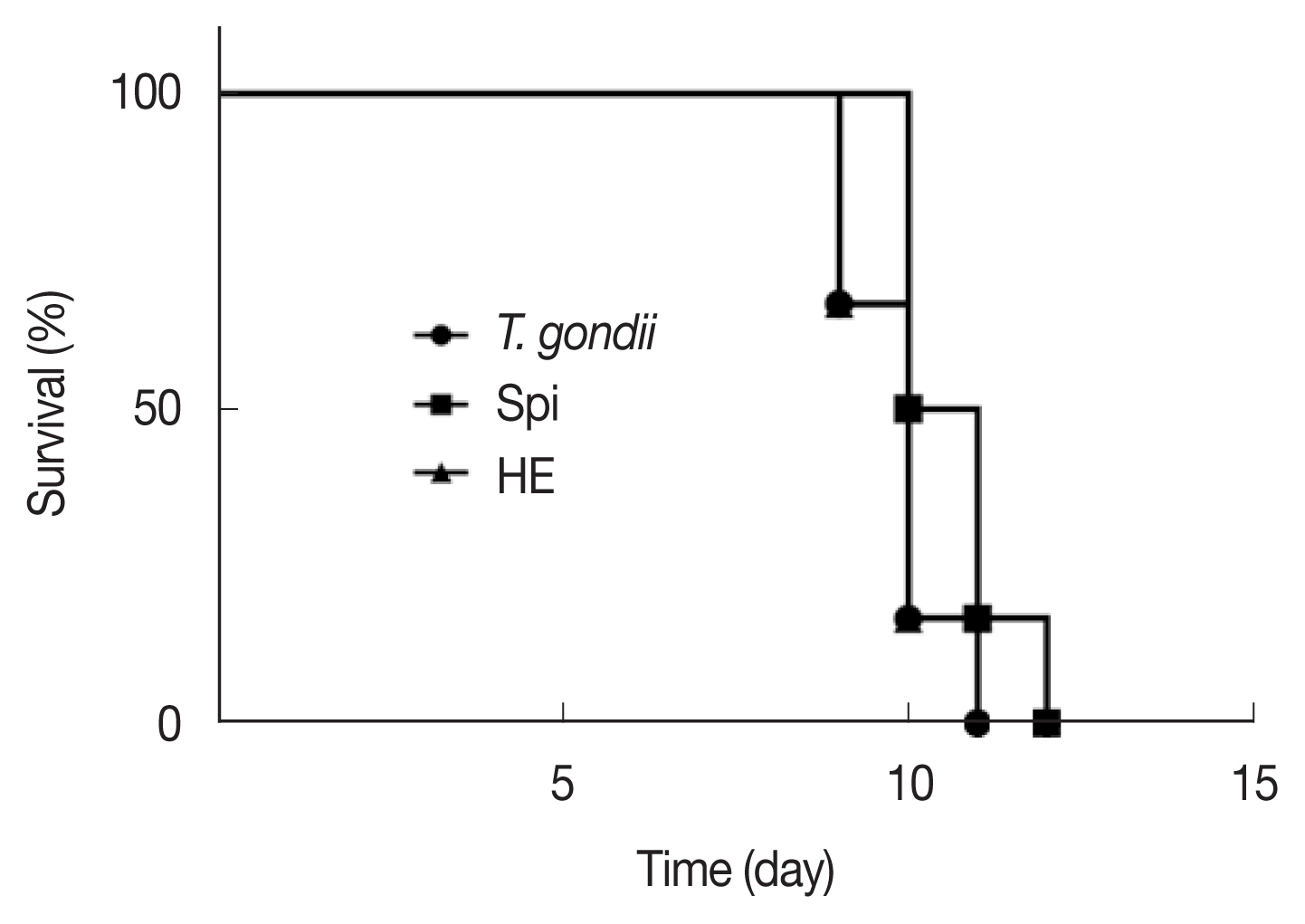
Survival rates of inoculated mice with tachyzoites of T. gondii, RH strain and treated with compounds, compared with control group (n=6).
In this study, we selected HE to study their anti-T. gondii activity in vitro. The conclusion is that HE exhibited superior activity compared to spiramycin, a drug used for the treatment of T. gondii infection. Simultaneously, HE was a good inhibitor of T. gondii in vivo and that it was effective in relieving the liver damage induced by T. gondii, and prolong the survival time of the mice to a certain extent. For this reason, HE has potential as an anti-T. gondii drug. Further studies are required to elucidate the mechanism of the action of HE, as well as to develop drug delivery systems for patients.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (No.81160409, 81960626).
Notes
No potential conflict of interest was reported by the authors.