Enterocytozoon bieneusi Genotypes and Infections in the Horses in Korea
Article information
Abstract
Enterocytozoon bieneusi is a microsporidian pathogen. Recently, the equestrian population is increasing in Korea. The horse-related zoonotic pathogens, including E. bieneusi, are concerns of public health. A total of 1,200 horse fecal samples were collected from riding centers and breeding farms in Jeju Island and inland areas. Of the fecal samples 15 (1.3%) were PCR positive for E. bieneusi. Interestingly, all positive samples came from Jeju Island. Diarrhea and infection in foals were related. Two genotypes (horse1, horse2) were identified as possible zoonotic groups requiring continuous monitoring.
Microsporidiosis caused by Enterocytozoon bieneusi is one of the most important diseases caused by ingesting food or water contaminated with spores and eggs [1]. It can cause life-threatening diarrhea in individuals with weakened immunity and is particularly related to chronic diarrhea in patients with AIDS [2]. E. bieneusi is challenging to observe through a microscope due to its small spore size of about 1 μm, various shapes, and in vitro culture method [3,4]. Therefore, for detecting and genotyping E. bieneusi, sequencing analysis by PCR of the ribosomal internal transcribed spacer (ITS) locus of E. bieneusi was used [3,5].
Enterocytozoon bieneusi was identified from livestock, companion animals, primates, wildlife, birds, and even wastewater [1,3]. By sequencing analysis of the ITS region of E. bieneusi, more than 500 genotypes have been identified and divided into 11 genetic groups [5,6]. Group 1 is the largest, while group 2 is the second largest group. These 2 groups are the most zoonotic-potential, and groups 3–11 mainly exhibit host specificity but lacks information [3,5]. More than 40 genotypes of E. bieneusi in equine have been identified, and classified into groups 1, 2, 6, and 10 [3].
Prevalence of E. bieneusi in equine was previously studied in several countries [7–11]. In Korea, several studies on E. bieneusi in other animals were performed [12–15] but no report from horses. The horse industry in Korea is expanding to horse breeding and leisure areas such as horse racing and horseback riding. Accordingly, the number of horses and people who encounter horses is increasing. Therefore, research on horse-related zoonotic pathogens, including E. bieneusi, is an important issue. This study surveyed the infection of E. bieneusi in horses and assessed its genotype.
From January 2019 to May 2021, 1,200 horse fecal samples were collected each from individual horses at riding centers and breeding farms, mainly in Jeju Island and other inland areas. This procedure was performed by experienced veterinarians, caused no harm to the animals, and did not require ethical approval. For data analysis, we requested to record basic information, such as age, sex, region, and collection date. The fecal samples with little or unclear information were classified as “unknown.”
Genomic DNA was extracted using a commercial kit from Qiagen (Hilden, Germany), all processes were performed according to the manufacturer’s protocol. The quality and quantity were measured using the Infinite 200 PRO NanoQuant plate reader (Tecan, Mannedorf, Switzerland). E. bieneusi was detected by nested PCR amplification of the ITS region using the AccuPower HotStart PCR Premix (Bioneer, Daejon, Korea). PCR was performed using primers and thermal cycling conditions used in previous studies [2,14]. The expected amplicon was about 390 bp [16]. All PCR products were electrophoresed in 1.5% agarose gel and stained with ethidium bromide.
For DNA sequencing and phylogenetic analysis, all PCR-positive samples were sequenced bidirectionally by Macrogen Co. (Daejeon, Korea). The nucleotide sequences were aligned, and consensus sequences were acquired using BioEdit 7.2.5 [17] and MEGA 7 [18]. Phylogeny was performed using MEGA 7 with the maximum-likelihood method. A phylogenetic tree was assessed by 1,000 replicate bootstrap analyses.
An overall E. bieneusi positive rate was 1.3% (15/1,200) (Table 1). It was low compared with the infection rate in other animals previously studied in Korea. The infection was reported in animals such as Korean water deer 53.6%, raccoon dogs 35.4%, calves 16.9%, cattle 14.9%, piglets 14.2%, bats 5.2%, cats 3.8%, and wild boar 2.6% [12–15,19–21]. In horses, E. bieneusi infection rate was lower in Korea than in other countries (Colombia 10.8%, China 30.9%, Czech Republic 17.3%, Turkey 18.7%) [8–10,22].
In this study, chi-square test was performed using the statistical package for social sciences (v.26, SPSS Inc., Chicago, Illinois, USA), and statistically significant results were obtained for all variables except sex (P<0.05). Most positive samples for age were from the ≤1 year group, 1 sample was from ≥11 years group, and 5 samples were of unknown age (P<0.001). Infection rate of these groups were 7.3% (9/123), 0.5% (1/223), and 2.5% (5/197), respectively. Similar cases were reported in Colombian equid species (≤1 year, 23.7%; >1 year, 2.5%) and in Chinese horses (≤1 year, 32.6%; >1 year, 30.6%) [8,9]. It was reported that the older group has a higher infection rate than the younger group in Czech Republic (≤3 years, 15.7%; >3 years, 19.6%) and in Turkey (≥4 years, 19.7%; 1–3 years, 16.3%) [10,22]. There was no difference in sex (female, 0.9%; male, 1.4%), which agrees with previous studies [8–10]. Association with diarrhea was higher in diarrheal than in normal ones (5.4% vs. 0.9%). E. bieneusi infection was confirmed in the spring (10/395; 2.5%) and summer (5/483; 1.0%). However, this does not mean that there was no infection in autumn and winter, but previous studies showed infection in the spring and summer [23–25]. According to regional data, all positive samples were confirmed in Jeju Island (15/923; 1.6%). Jeju is the largest island on the Korean peninsula and located in the southernmost part. Its climate is warmer than in mainland Korea. However, number of positive fecal samples was small, and more than half of the Korean horses were bred in Jeju [26]. Therefore, whether E. bieneusi infection of horses is specific to the Jeju region needs further study.
By sequencing, 3 representative sequences were obtained from 15 PCR-positive products and submitted to GenBank under accession numbers MW753227–MW753229. A phylogenetic tree confirmed 2 genotypes, horse1 and horse2, belonging to groups 1 and 6 (Fig. 1). Group 6 comprised of 8 samples, while group 1 of 7 samples. There was no mixed infection of the E. bieneusi genotypes.
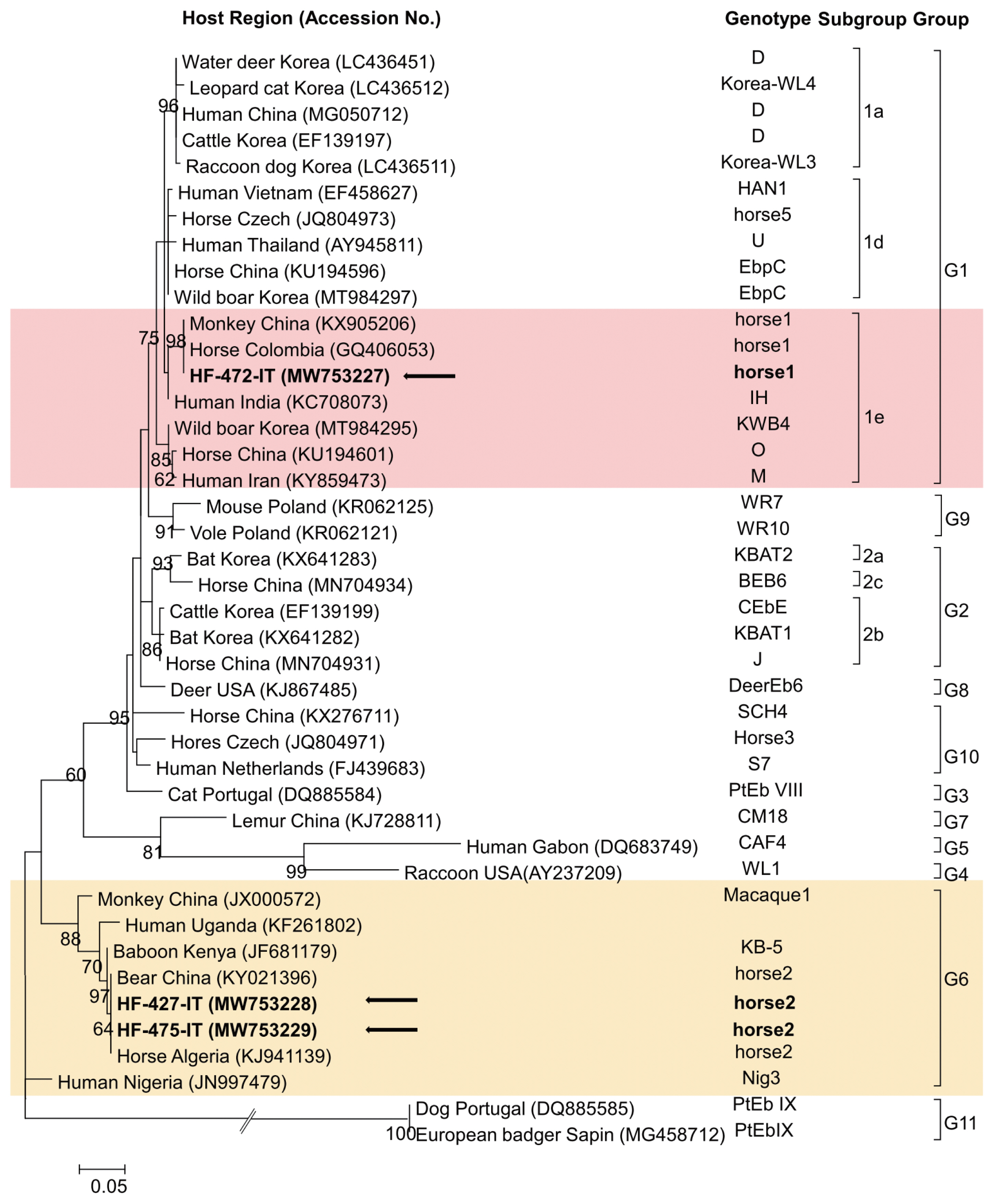
A phylogenic tree figured based on the internal transcribed spacer sequence of Enterocytozoon bieneusi detected from the horses. Sequences identified in this study are indicated using arrows. Genotypes (groups and subgroups) are shown on right.
Group 1 is the largest group divided into 9 subgroups (1a–1i), with more than 300 genotypes identified [3], in various animals, including horses and humans. Group 1 is common in horses. Horse E. bieneusi genotypes were 1a, 1b/c, 1d, 1e, and 1g subgroups [3]. In horses, E. bieneusi was also identified in group 6, and genotypes clustered into groups 2 and 10 [8,10,27]. This study identified the E. bieneusi genotypes clustered into subgroup 1e (MW753227) and group 6 (MW753228, MW753229) (Fig. 1). Subgroup 1e was identified several times in other animals in Korea [12,19,21], but the group 6 genotype was identified for the first time in this study. The sequence identified as subgroup 1e was 100% identical to the sequence reported in Colombian horse (GQ406053) and the Chinese Monkey (KX905206), and 98.9% similar to E. bieneusi in Indians (KC708073). This showed 97.4% sequence similarity with genotype 1e in Korean wild boar (MT984295), suggesting low level of host specificity and zoonotic potential [3].
Group 6 genotype has been found in animals (equine, ruminants, nonhuman primates, rodents) and humans [7,28–31]. This genotype has strong host specificity since it has been identified only in the originally reported host species, but new hosts for the same genotype have recently been identified [3,30,32]. Therefore, the group 6 subgenotypes were suggested with zoonotic potential. The group 6 sequence identified in this study (MW753228) showed 100% identity with the sequence identified in Chinese bear (KY021396) and 99.7% identity to sequence in the Algerian horse (KJ941139). The horses may play a potential role as a reservoir for zoonotic transmission since they showed 97.4% sequence similarity to E. bieneusi in the Ugandans (KF261802).
This is the first study of E. bieneusi infection in horses in Korea, providing basic public health data related to E. bieneusi infection pattern and rate. The results indicated that young age and diarrhea were associated with infection. Although the infection rate was low, a positive test result was observed only in the Jeju Island. This research is needed to be continued since both groups had zoonotic transmission potential.
ACKNOWLEDGMENTS
This work was supported by grants from the Animal and plant quarantine agency (B-1543069-2019-21-03), Korea, and from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2016R1D1A1B02015366).
Notes
The authors declare no conflict of interest related to this study.

