Antimalarial Efficacy of Aqueous Extract of Strychnos ligustrina and Its Combination with Dihydroartemisinin and Piperaquine Phosphate (DHP) against Plasmodium berghei Infection
Article information
Abstract
The development of drug resistance is one of the most severe concerns of malaria control because it increases the risk of malaria morbidity and death. A new candidate drug with antiplasmodial activity is urgently needed. This study evaluated the efficacy of different dosages of aqueous extract of Strychnos ligustrina combined with dihydroartemisinin and piperaquine phosphate (DHP) against murine Plasmodium berghei infection. The BALB/c mice aged 6–8 weeks were divided into 6 groups, each consisting of 10 mice. The growth inhibition of compounds against P. berghei was monitored by calculating the percentage of parasitemia. The results showed that the mice receiving aqueous extract and combination treatment showed growth inhibition of P. berghei in 74% and 94%, respectively. S. ligustrina extract, which consisted of brucine and strychnine, effectively inhibited the multiplication of P. berghei. The treated mice showed improved hematology profiles, body weight, and temperature, as compared to control mice. Co-treatment with S. ligustrina extract and DHP revealed significant antimalarial and antipyretic effects. Our results provide prospects for further discovery of antimalarial drugs that may show more successful chemotherapeutic treatment.
INTRODUCTION
Malaria is a disease caused by Plasmodium, which belongs to apicomplexan parasites. The disease is transmitted by mosquito vectors. Several Plasmodium species, such as P. vivax, P. falciparum, P. malariae, P. ovale, and P. knowlesi cause human infections. Those parasites are widely distributed worldwide. Malaria is still one of the most highly pathogenic diseases and remains a significant public health problem. Malaria mortality was reported in more than 60% of infected children worldwide [1].
Malaria situation has worsened due to the emergence of Plasmodium species that are resistant to antimalarial drugs. This could lead to more obstacles that hinder disease control [2]. The World Health Organization recommends the artemisinin-based combination therapy for treating malaria [1]. Natural compounds can be used as alternative antimalarial drugs that can be utilized in artemisinin-based combination therapies [3]. The combination could enhance the potency of natural extracts and minimize parasite resistance.
In Indonesia, many natural compounds are used as alternative medicines. One of these natural compounds is Strychnos ligustrina. The aqueous extract of S. ligustrina demonstrated an antimalarial activity in mice. Two compounds, including flavonoids and alkaloids, play important roles in antimalarial activity. Previous studies have shown that ethanol extract of S. ligustrina wood was successively exhibiting potent antimalarial activity against P. falciparum multiplication. Further studies have shown that the antimalarial activity of aqueous extract of S. ligustrina extracted from maceration with water was higher than that of ethanol extract. Brucine (indole alkaloid) was the dominant compound in both the aqueous and ethanol extracts. The aqueous extract contains a higher level of brusine (24.9%) than the ethanol extract (11.6%) [4,5].
Therefore, this study aimed to evaluate the efficacy of aqueous extract of S. ligustrina and combination treatment of dihydroartemisinin and piperaquine phosphate (DHP) and aqueous extract of S. ligustrina against Plasmodium berghei in an infected mice model. Our findings demonstrated that antimalarial compounds of S. ligustrina may improve antimalarial efficacy when combined with DHP.
MATERIALS AND METHODS
Ethical consideration
The Animal Ethics Commission of Bogor Agricultural University has approved the in vivo experiments on mice (accession number: 154-2019 IPB).
Plant sample
Strychnos ligustrina wood samples were obtained from Dompu West Nusa Tenggara, Indonesia. The collected plant sample was identified by the Laboratory of Silviculture, Faculty of Agriculture, University of Mataram.
Preparation of crude extract
The extraction was carried out on a pilot project scale at Fits Mandiri Company, Bogor, Indonesia. A total of 31 kg of S. ligustrina wood shavings (moisture content of 11.8%) was macerated with distilled water (5% Brix: 5 g of solid in 100 g of solution). Extraction was carried out twice. The extracted filtrate was then dried using a spray dryer to produce an extract powder. The standard compounds used for the high-performance liquid chromatography (HPLC) analysis were strychnine and brucine (Sigma, Darmstadt, Germany).
High-performance liquid chromatography (HPLC) analysis
Brucine and strychnine components in S. ligustrina extract were quantified using reverse-phase HPLC with a C18 column. Acetonitrile and water were employed as the mobile phase, using a 5–95% acetonitrile gradient elution method for 30 min. The intensity of the chemical at the concentration used was measured at wavelength 254 nm.
Parasite
The P. berghei Antwerpen-Kasapa (ANKA) strain was obtained from the National Institute of Health Research and Development, Indonesian Ministry of Health. The parasites were passaged by inoculating intraperitoneally from the infected mice to the healthy mice every week.
In vivo antimalarial inhibition test
The growth inhibitory effect of antimalarial drugs was determined using mice infected with P. berghei. The BALB/c mice aged 6–8 weeks were divided into 6 groups, consisting of 10 mice each. Groups A and B comprised healthy and infected-untreated mice, respectively. Group C, used as the control group, received 222 mg/kg body weight (BW) of DHP. Group D received 300 mg/kg BW of aqueous extract of S. ligustrina. Group E received a combination of 111 mg/kg BW of DHP and 200 mg/kg BW of aqueous extract of S. ligustrina, and Group F received 111 mg/kg BW of the control drug (DHP). The drugs were administrated orally. The treatment was started when the average parasitemia reached 10% on day 6 post-infection (p.i.). The treatment was started and continued for 4 consecutive days (days 6 to 10 p.i.).
Inoculation of P. berghei
Before starting the experiment, the parasites were obtained from the frozen stock at −80°C and injected intraperitoneally to the mice. At least 3 times of passage of the parasites from the infected mouse to the healthy mouse were maintained. Subsequently, after the passages, the donor mouse was prepared by injecting parasites intraperitoneally. Parasitemia was monitored by using Giemsa-stained blood smears every 2 days. When the parasitemia reached approximately 20%, the mice were euthanized, and blood was collected via cardiac puncture. Blood was diluted with phosphate buffered saline (PBS); 0.5 ml of blood contained 2×106 P. berghei infected red blood cells (RBCs). Each group, except for group A, received 0.5 ml of infected RBCs intraperitoneally [6].
Monitoring of parasitemia
Parasitemia was monitored every 2 days by preparing blood smears on a microscope slide. The slides were dried, fixed, and stained with 10% Giemsa [7]. The thin blood smears were examined using a microscope under 1,000×magnification. The parasites were counted for every 1,000 RBCs.
Monitoring of hematological profiles, body weight, and temperature
The body weight and temperature were measured every 2 days during the experimental period. The hematological profiles were evaluated every 4 days using a hematological analyzer (Medically Automatic Hematology Analyzer Mindray BC-2800 Vet, Shenzhen, China). All parameters were assessed for 20 days post-infection.
Data analysis
All variables were analyzed using the Student’s t-test. The difference in each variable between the untreated and treated groups was considered statistically significant when P<0.05.
RESULTS
The chromatograms of brucine and strychnine standard compounds were found with retention times of 10.6 and 11.7 min, respectively (Supplementary Fig. S1). Using the same separation process, extract analysis was done on a sample concentration of 5,080 ppm. Since identical retention times were found in the standard chromatograms for brucine and strychnine, retention time similarity analysis revealed that the extract samples contained brucine and strychnine (Supplementary Fig. S2). By comparing the peak area of brucine and strychnine retention times on the standard chromatograms and extract samples, the quantitative results demonstrated that amount of brucine was higher than that of strychnine. The concentrations of brucine and strychnine were 13.5 and 9.8 mg/g extract, respectively (Table 1).
In vivo antimalarial inhibition test
The aqueous extract of S. ligustrina had an antiplasmodial activity. This was observed from the parasitemia and inhibition activity, which are comparable to other groups. Overall, the peak of parasitemia was observed on day 7 p.i. for groups B (infected-untreated), C (222 mg/kg BW of DHP), and E (combination treatment of 200 mg/kg BW of aqueous extract of S. ligustrina and 111 mg/kg BW of DHP), while group D (300 mg/kg BW of aqueous extract of S. ligustrina parasitemia) peaked on day 6 p.i. The highest percentages of parasitemia for groups B, C, D, E, and F were 21.5%, 19.5%, 22.1%, 11.5%, and 12.5%, respectively (Fig. 1).
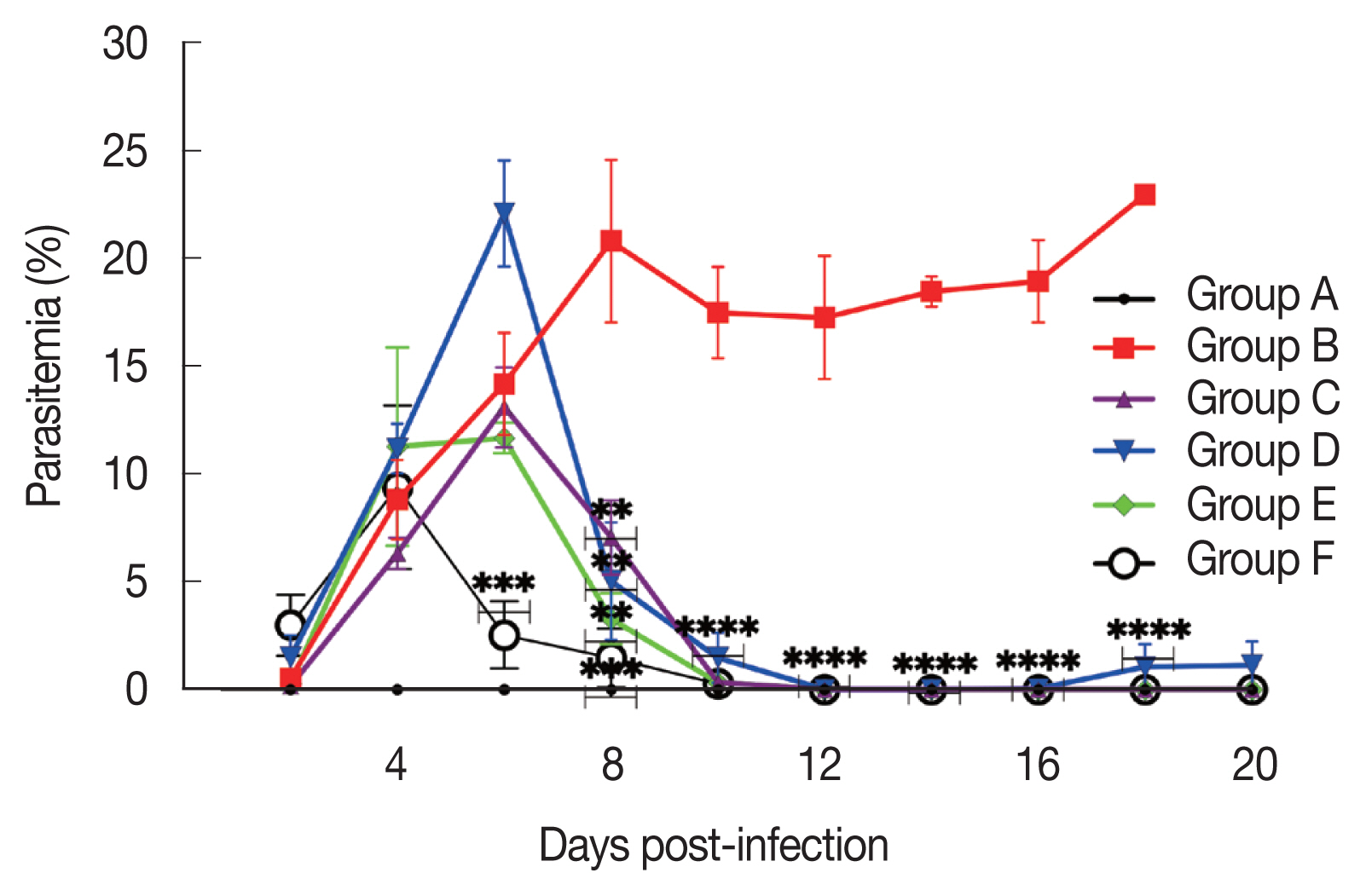
Effect of drug treatment on the growth of Plasmodium berghei in mice. The parasitemia rate after treatment with 300 mg/kg body weight (BW) of mono-extract of Strychnos ligustrina, 111 mg/kg BW of dihydroartemisinin and piperaquine phosphate (DHP), and their combination therapy. The treatment regime was started from day 6 to 10 post-infection. The data are shown as a mean from 2 experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, compared to the group B by student t-test.
Hematology profiles
Group D, which received 300 mg/kg BW of aqueous extract of S. ligustrina, showed a decrease in RBC count and hemoglobin and hematocrit levels, as compared to group B (untreated-uninfected). Moreover, no statistically significant difference (P>0.05) was found between groups D and A (negative control) (Fig. 2).
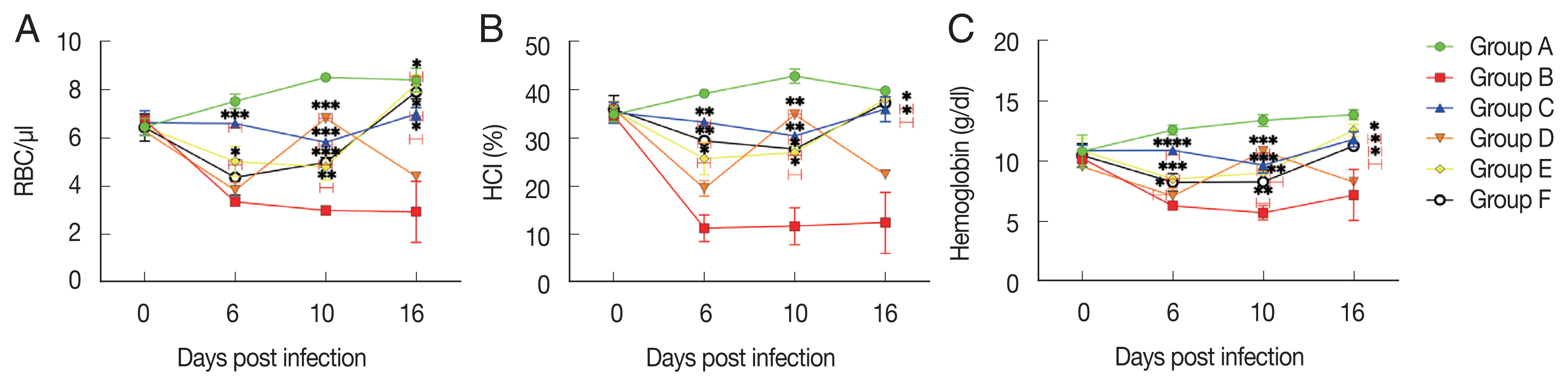
Hematology profiles of mice from day 2 to 16 post-infection. Hematology profiles, including hematocrit (A), hemoglobin (B), and red blood cells (C) were monitored every 4 days. All data obtained from the selected mouse are presented as the mean±SD. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, compared to the group B by student t-test.
Survival rate
The treated groups (C, D, E, and F) were survived until 20 days p.i. The 20-day survival rates after infection were found to be 34%, 42%, 38%, and 40%, respectively, in animals treated with the extract alone, control drug, combination treatment, and half dose of the control drug (Fig. 3).
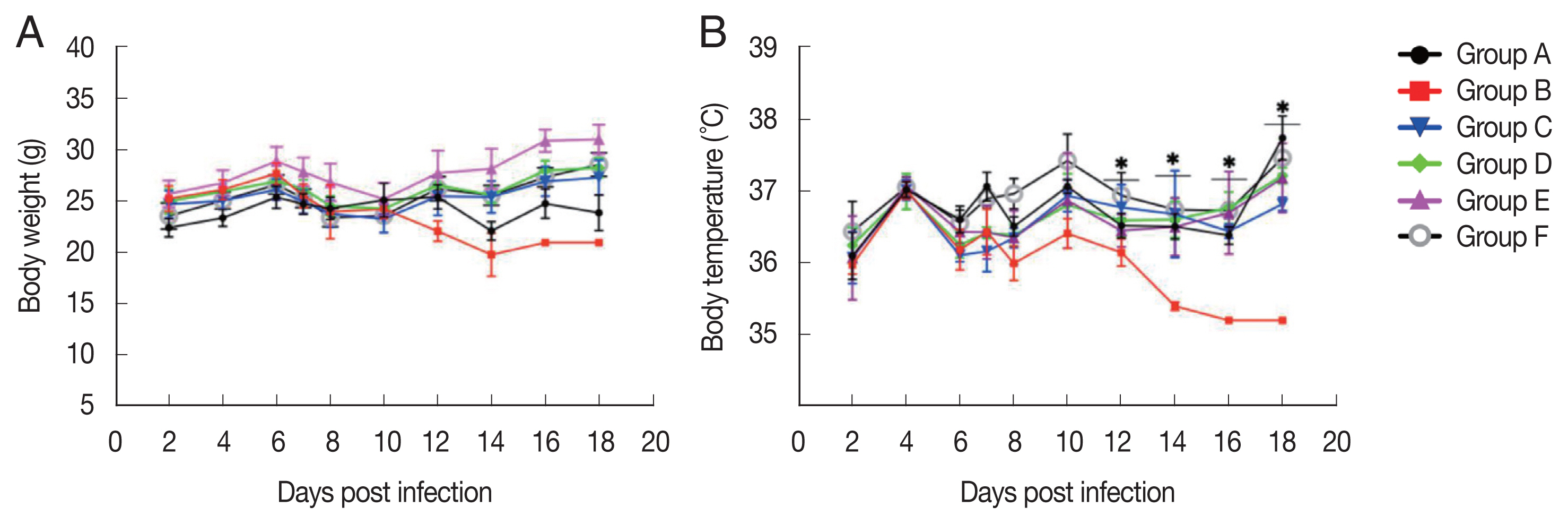
Body weight and temperature
The treated group did not show parasite-induced weight reduction, as compared to group A (negative control). In contrast, infected-untreated group showed a weight reduction, especially on days 12 to 16 p.i. The body temperature of the infected-untreated group dramatically decreased from 6 to 18 days p.i. However, the treated-infected group maintained their temperature, as compared to the healthy mice (Fig. 4).
DISCUSSION
The development of drug resistance is one of the most severe concerns to malaria control since it leads to an increase in the rates of malaria morbidity and death. The resistance to the artemisinin and non-artemisinin components has recently arisen in the Southeast Asian regions [1]. A new candidate drug with a reliable antiplasmodial activity, such as an herb extract, is urgently needed [8].
Our findings showed that the single usage of the extract and combination treatment inhibited the growth of P. berghei by 74% and 94%, respectively, as compared to the untreated group. The mice treated with the extract alone and combination therapy showed the highest percentage of parasitemia on days 7 and 8, respectively. Even though single usage of the extract and combination treatments were less effective than DHP against murine P. berghei infection, they showed better inhibition than the control drug. Interestingly, on day 7 p.i., the group treated with 300 mg/kg BW of aqueous extract of S. ligustrina had the highest parasitemia rate (22.1%). The parasitemia rate of the aqueous group was dramatically reduced by 12% on day 8 p.i. (Fig. 1). This finding showed that a single extract of S. ligustrina contained a compound that inhibited the multiplication of P. berghei. Since a single extract of 300 mg/kg BW was administered, it could be classified as having reliable antiplasmodial efficacy [8]. Previous studies also reported that similar plants belong to Strychnos had antimalarial activity [9–12]. In this study, single extract treatment and combination treatment inhibited P. berghei proliferation. In addition, the aqueous extract improved hematological profiles, body weight, and temperature. Our study showed that aqueous extract of S. ligustrina contained high antiplasmodial active compounds, including brucine and strychnine, which is consistent with the previous studies [4,5,13]. Besides the antiplasmodial activity, brucine also has anti-inflammatory and antipyretic activities [14]. In addition, brucine significantly reduced the reactivity induced by the peripheral nerve heat and mechanical stimulations by directly lowering sodium channel excitability [15]. Brucine may also be beneficial to reduce pain and inflammation, thus improve temperature stability. S. ligustrina contains a strychnine, which has a toxic effect; however, the concentration used in this study was below the safety limit (9.8 mg/g or 1.2 mg/kg when converted at a 300 mg/kg body weights for 4 days). S. ligustrina extract may be a safe antimalarial compound as the dose is lower than the LD50 of strychnine in mice with 2 mg/kg [16,17].
In conclusion, treatment of a single extract of S. ligustrina and combination therapy revealed significant antimalarial and antipyretic effects against murine P. berghei infection model. Most notably, combining the aqueous extract with DHP may result in a more successful chemotherapeutic treatment. The mode of action of a S. ligustrina extract and the efficacy of these derivatives against Plasmodium parasites await further study.
Supplementary Information
ACKNOWLEDGMENTS
This research was funded by the Directorate of Higher Education of the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia with the IPB Higher Education Research Grant scheme 2021 (contract No. 1/EI/KP.PT.PTNBH/2021) for funding support for this research.
Notes
The authors declare no conflict of interest related to this study.