Phylogenetic Characteristics of Fasciola hepatica Isolated from a Korean Patient
Article information
Abstract
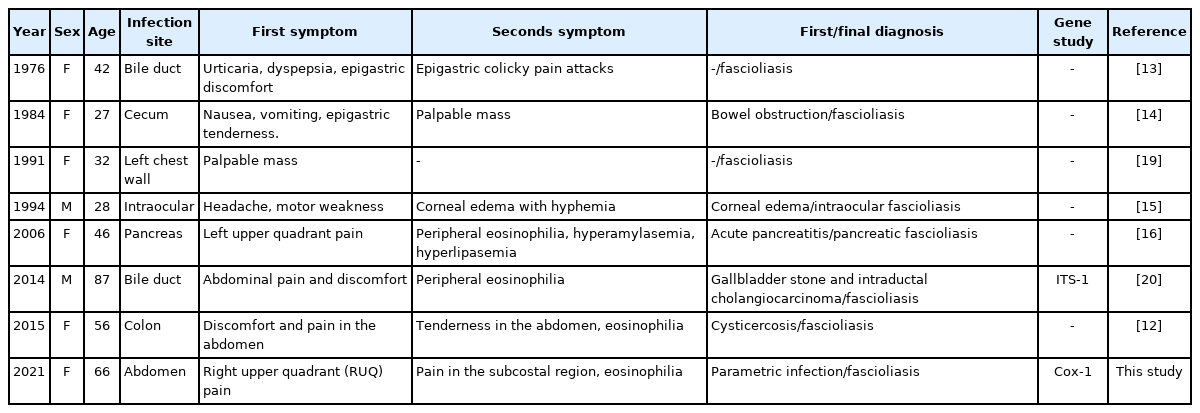
Fascioliasis is a parasitic infection caused by liver flukes. Although several cases have been reported in Korea, phylogenetic analysis of isolates is lacking. In this study, a 66-year-old woman with right upper quadrant (RUQ) abdominal pain was diagnosed as fascioliasis involving abdominal muscle by imaging study. She received praziquantel treatment, but symptoms were not improved. Lateral movement of the abscess lesion was followed. Trematode parasite was surgically removed from the patient’s rectus abdominis muscle. The fluke was identified as Fasciola hepatica based on sequence analysis of 18S rDNA. To determine the phylogenetic position of this Fasciola strain (named Korean Fasciola 1; KF1), the cox1 gene (273 bp) was analyzed and compared with the genes of 17 F. hepatica strains isolated from cows, sheep, goats, and humans from various countries. Phylogenetic analysis showed that KF1 was closely related with the isolates from China goat.
Fascioliasis in livestock and humans are caused by the genus Fasciola [1]. Fasciola spp. are prevalent in temperate climates. Fascioliasis is one of the neglected zoonotic diseases declared by the World Health Organization (WHO) [2]. Incidence of fascioliasis has been significantly increased since 1980, affecting approximately 2.4 million people globally each year. In humans, infection occurs through ingestion of contaminated water containing Fasciola metacercaria(e). The larvae pass through the stomach, break through the duodenal barrier, penetrate the abdominal cavity, and enter the hepatobiliary system. When the ingested fluke moves to the bile duct through the abdominal cavity and liver parenchyma, extensive bleeding and inflammation occur, causing thickening and expansion of the bile duct and gall bladder [3,4]. The symptoms and signs of human fascioliasis occur in 2 stages. The hepatic stage of the disease occurs when juvenile Fasciola enters the liver and begins to migrate to the biliary root canal; this is referred to as the acute or invasive stage. This stage occurs 1–3 months after the ingestion of metacercariae, in which typical symptoms and signs include fever, hives, hepatomegaly, and pain in the right hypochondrium. When the worm infects biliary tract, cholangitis, cholestasis, right upper quadrant (RUQ) pain, and eosinophilia are usually developed [5–7].
Genetic diversity of Fasciola populations has been demonstrated via molecular analysis of their nuclear and mitochondrial DNAs (mtDNAs). The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis of mtDNA revealed 52 unique sequences in 221 flukes [8]. A study on Fasciola isolates from livers of sheep and cattle suggested grouping of these isolates into 3 main lineages (F. hepatica, F. gigantica, and F. indica), using the cox1 (encoding cytochrome c oxidase subunit) gene. Ai et al. [1] also inferred phylogeny using the cox1, which is a useful marker for studying the genetic differentiation and phylogenetic relationship of Fasciola.
Infection with Fasciola spp. has been reported in East Asia, including Korea (Table 1) and Japan, while few molecular taxonomic studies have been conducted on Korean species [10]. In this study, the genetic characteristics of Fasciola isolated from a Korean patient were compared with those of several previously reported isolates using the cox1 gene sequencing and multiple alignment methods.
A 66-year-old Korean woman living in Pusan visited the Pusan National University Hospital on July 11, 2021, with an abdominal mass. She had been suffered from hypothyroidism. In June 2021, she was diagnosed with a hepatic abscess by computed tomography (CT) performed during an evaluation of RUQ pain at another hospital. The symptoms improved by symptomatic treatment and she was discharged after administration of antibiotics (ceftriaxone and metronidazole) (Fig. 1A). She visited again our hospital due to recurrence of the RUQ pain. The mass was palpable in the abdominal cavity, we checked the mas with CT. Some swelling and cell infiltration was confirmed, and parasitic infection was suspected (Fig. 1B). She received praziquantel (25 mg/kg 3 days). Most fascioliasis patients showed eosinophilia and abdominal distension [11]. Abdominal distension along with eosinophilia was also confirmed in this patient. In August 2021, liver abscess was percutaneously drained, after which she was discharged. However, she complained of new pain in the subcostal region (Fig. 1C). Praziquantel treatment were maintained, but her symptoms did not improve. New multiple abscesses were found below the previous lesion on abdominal CT. Compared to the previous CT, a lateral movement of the abscess was observed with maximum size of 3.4 cm (Fig. 1D). The CT showed abdominal distension. The laboratory test showed eosinophilia (7.3%, normal range 0–6.9%) with low number of segmented neutrophil (38.8%, normal range 40.0–70.4%). The mean corpuscular hemoglobin concentration, eosinophil count, and erythrocyte sedimentation rate increased, and the levels of seg neutrophils decreased. A biopsy revealed a leave-like parasite. The length was approximately 1.7–1.8-long, and the width was 0.5–0.8-long. The morphology of the posterior testis of the F. hepatica was observed under a microscope. The fluke did not have hooks or spines but were characterized by suckers. The acetabulum wis located at the anterior part of the worm (Fig. 2A). The granuloma contained acute and chronic inflammatory reactions accompanied by numerous eosinophilic invasive micronecrosis.
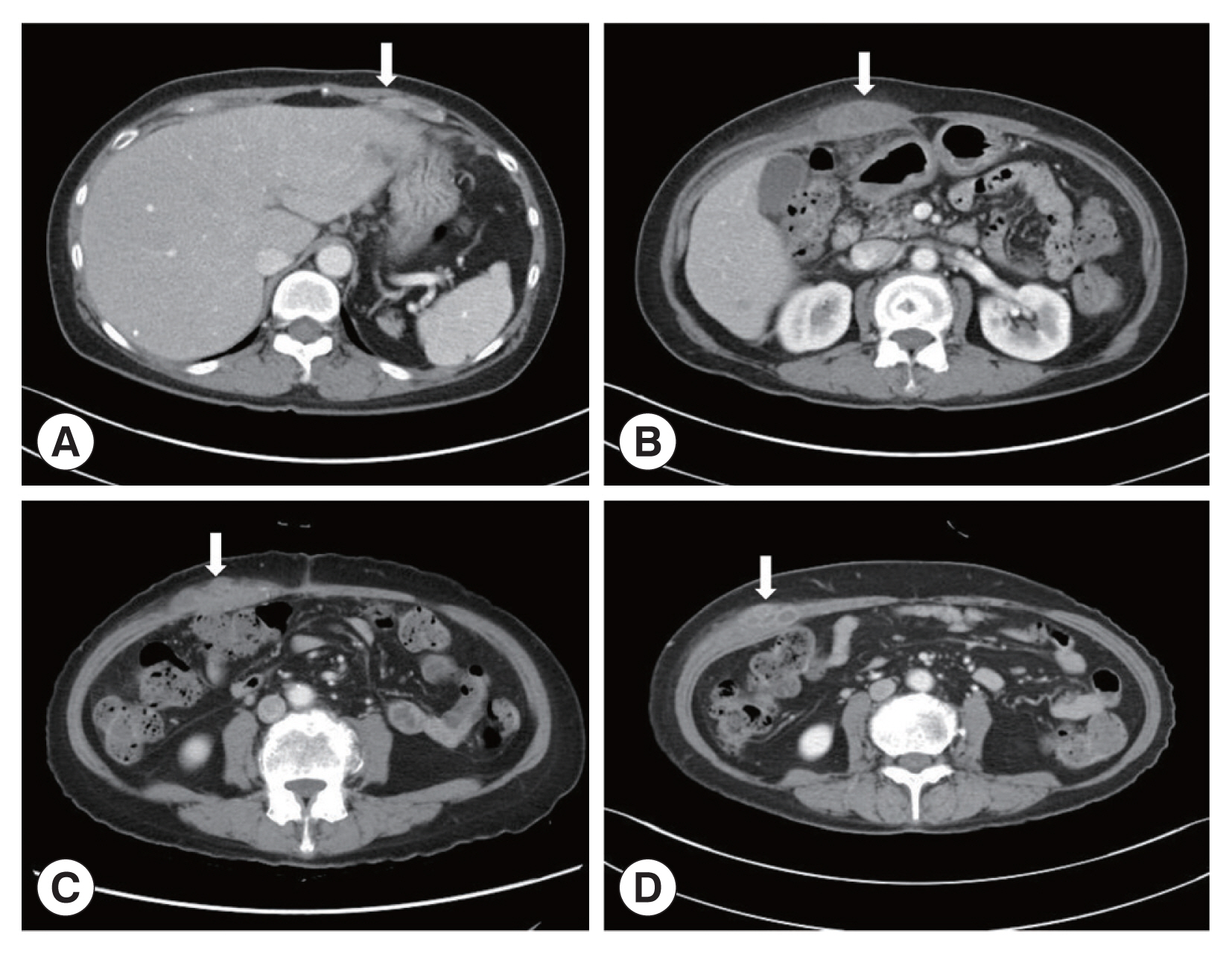
Abdominal computerized tomography (CT). (A) CT performed at the time of admission for evaluation of pain in the right upper quadrant (RUQ). (B) RUQ pain recurred after treatment of liver abscess. CT was taken to evaluate the abdominal wall mass. (C) Abdominal CT performed with new pain in the subcostal area. (D) There was no improvement after maintenance treatment with praziquantel. CT was taken again at Pusan National University Hospital. The arrows indicated a mass lesion.
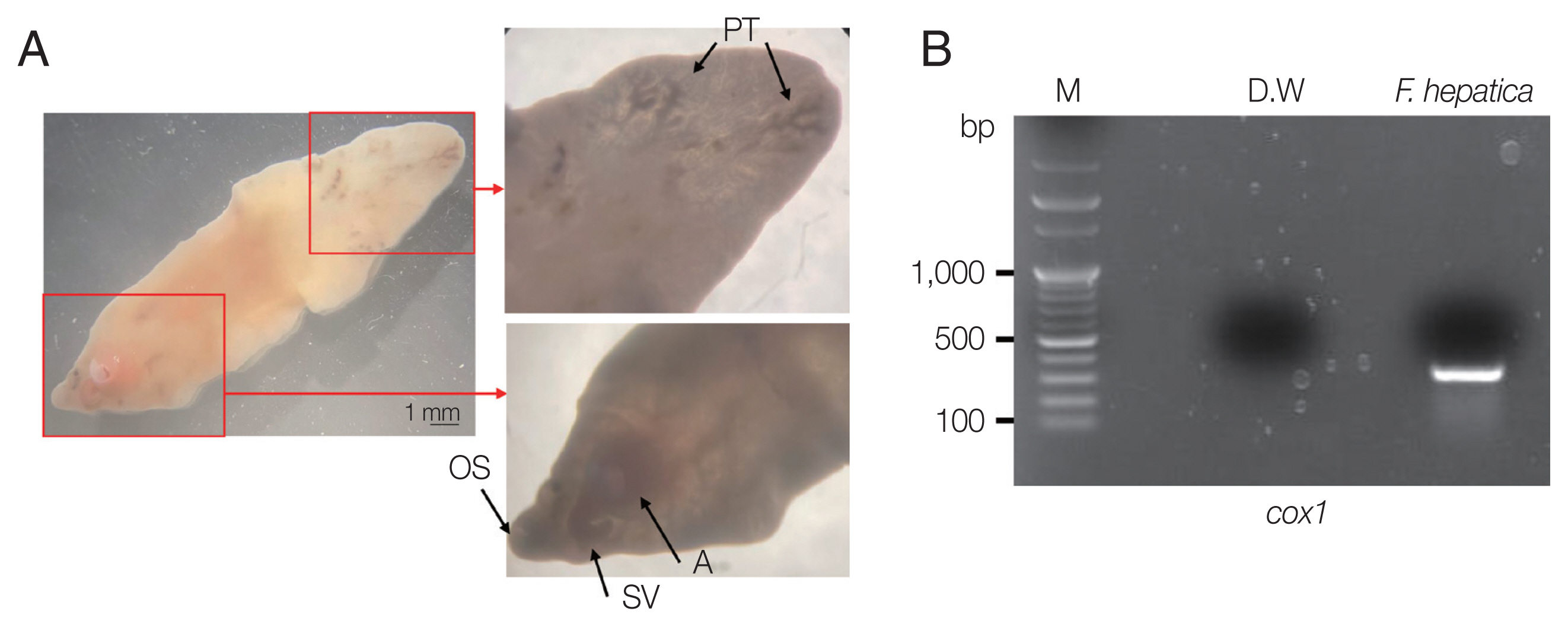
Morphological characteristics of liver fluke and electrophoretic analysis of PCR products. (A) Morphological characteristics of F. hepatica isolated from the patient. OS, oral sucker; A, acetabulum (ventral sucker); SV, seminal vesicle; PT, posterior testis. (B) Cox1 partial PCR product (M, 100-kb ladder).
This patient came to our hospital with a liver abscess, but was diagnosed with abdominal muscular fascioliasis invoking RUQ pain. A parasitic infection caused by Fasciola hepatica was confirmed by histological observation of biopsied material. F. hepatica usually thrives in the hepatobiliary system, it often migrates aberrantly and causes ectopic fascioliasis [12]. Ectopic fascioliasis in the various sites, such as the cecum, ascending colon, brain, eyes, spine, subcutaneous tissue, neck, and inguinal lymph nodes, have been reported in Korea [13–15]. In 2006, a very rare case of pancreatic infection was also reported in Korea [16]. F. hepatica infection should be suspected in the differential diagnosis of parasitic infection based on the patient’s dietary history, eosinophilia, symptoms, and results of ELISA tests.
To molecularly identify F. hepatica, PCR was performed on worm’s DNA sample. We also establish the phylogenetic classification of this specimen using the F. hepatica 18S rRNA and cox1 gene sequences. Their genetic mutations were compared with 17 previously reported strains. Amplification of the cox1 gene was performed using primers cox1 F 5′-TTTGCCTGG GTTTGGAGTTA-3′ and cox1 R 5′-CCACACAACAGGATCCCATA-3′. The PCR conditions were optimized according to previous reports [17]. Through PCR amplification, a target band with a length of approximately 273 bp was obtained for the cox1 gene (Fig. 2B). DNA sequencing was performed using Cosmo Genetech (Seoul, Korea). Nucleotide sequences were grouped according to host species (cattle, sheep, goat, and Homo sapiens) and region (South Africa, Tunisia, Austria, Ecuador, France, and China). Isolates and reference sequences were used for multiple alignments and phylogenetic distances were calculated using MEGA version 6.0. Phylogenetic trees were constructed using the neighbor-joining method in the MEGA version 6.0 using cox1 partial sequences isolated from a Korean patient (KF1) with 17 previously reported isolates. As shown in Fig. 3, the phylogenetic tree clearly separated these homologs according to the host and region. KF1 was found to be closely related to F. hepatica from goats in China, and the genetic characteristics of most of the isolates were geographically related, with no relationship was observed along with host differences.
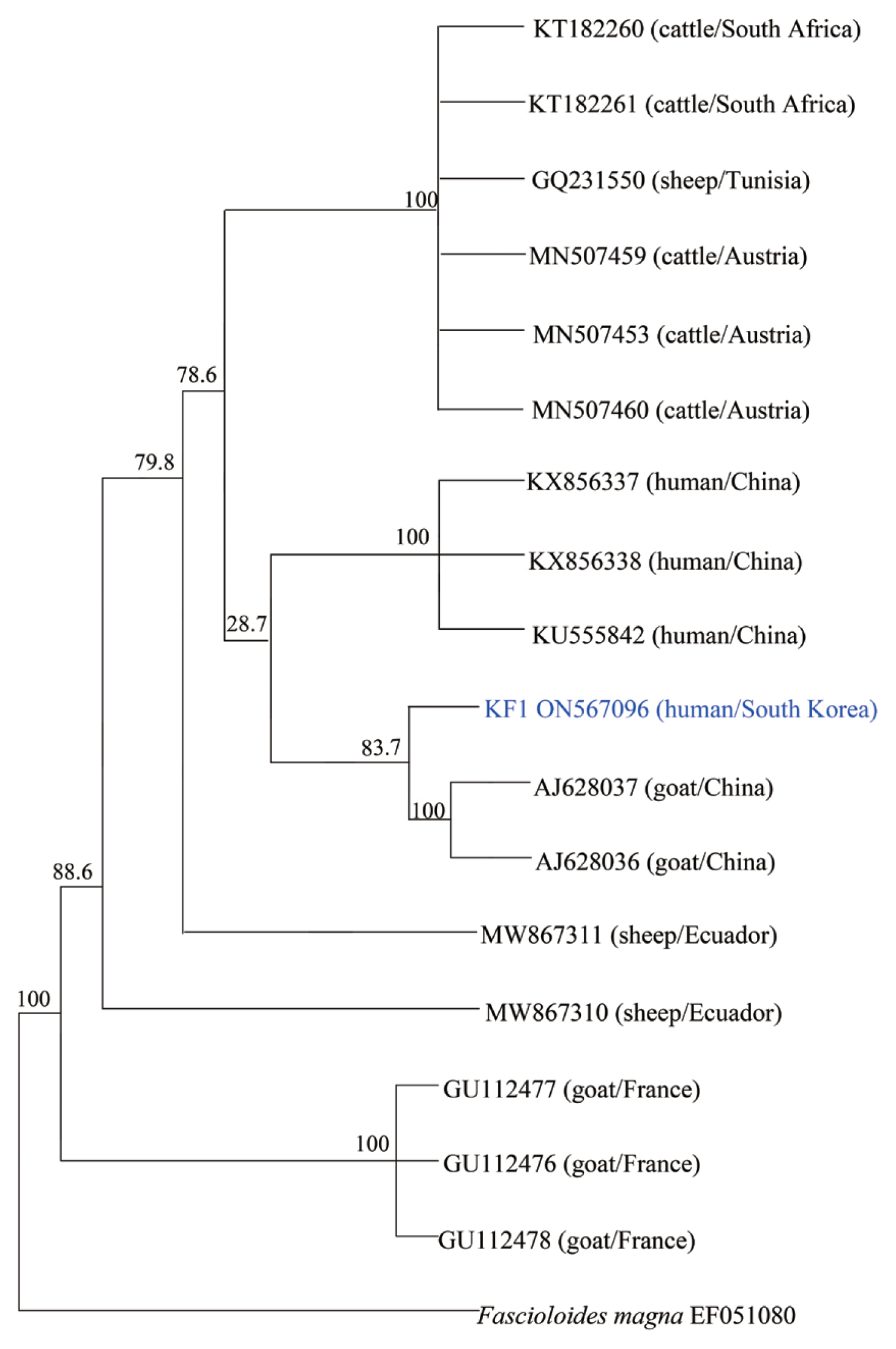
Phylogenetic tree of F. hepatica isolates generated using cox1 partial gene sequences. All of F. hepatica was represented by Genbank accession numbers. The genetic relationship between F. hepatica KF1 (ON567096) and 17 corresponding reference sequences was analyzed by the neighbor-joining method using MEGA, version 6.0.
Fascioliasis is generally diagnosed by detection of the characteristic eggs in feces. However, at low levels of infection, the sensitivity is very low even after repeated stool examinations. Serological tests are appropriate for diagnosing patients at the invasive stage, as they allow for early detection of infection. Anti-Fasciola antibodies can be detected 2–4 weeks after infection [6,18].
A limitation related to the interpretation of the phylogenetic results in this study is that only one Korean isolate was used for the analysis. Phylogenetic analysis has revealed that goat cattle, and sheep share F. hepatica strain that can transmits to humans. In the context of the prevention of human fascioliasis, the pattern and phylogenetic aspects of parasitic infections appear to be very important. In Korea, there was genetic confirmation of F. hepatica, but there were no related data in the phylogenetic analysis. A study on the distribution of F. hepatica is needed for future epidemiological investigations of domestic infections.
Notes
The authors declare that they have no conflict of interest.