Abstract
Balamuthia mandrillaris amebic encephalitis (BAE) can cause a fatal condition if diagnosis is delayed or effective treatment is lacking. Patients with BAE have been previously reported in 12 provinces of China, with skin lesions being the primary symptom and encephalitis developing after several years. However, a significantly lower number of cases has been reported in Southwest China. Here we report an aggressive BAE case of a 64-year-old woman farmer with a history of skin lesions on her left hand. She was admitted to our hospital due to symptoms of dizziness, headache, cough, vomiting, and gait instability. She was initially diagnosed with syphilitic meningoencephalitis and received a variety of empirical treatment that failed to improve her symptoms. Finally, she was diagnosed with BAE combined with amebic pneumonia using next-generation sequencing (NGS), qRT-PCR, sequence analysis, and imaging studies. She died approximately 3 weeks after the onset. This case highlights that the rapid development of encephalitis can be a prominent clinical manifestation of Balamuthia mandrillaris infection.
-
Key words: Balamuthia mandrillaris, amebic encephalitis, amebic pneumonia, Southwest China
Introduction
Balamuthia mandrillaris ameba (
B. mandrillaris), a worldwide distribution of single-celled protozoa, can live independently in hot and dry soil, dirty dust, or water environments [
1–
5]. This free-living organism was first identified in a mandrill brain autopsy at the San Diego Wildlife Park, United States, in 1986 [
6] and it has been known to cause
B. mandrillaris amebic encephalitis (BAE) in dogs, horses, bats, and primates [
7,
8].
B. mandrillaris has been mainly reported in the United States (Southwest) and South America, especially among Hispanic populations [
9]. It is speculated that a hot and dry environment might be the type of environment in which Balamuthia thrives [
9,
10]. However, a study on the soil gene search of
B. mandrillaris demonstrated that this type of opportunistic ameba can spread to wider climatic areas, even in areas characterized by blizzards [
11]. Consequently, during the last few years, several Asian regions, such as South Korea [
12,
13], Japan [
11,
14], Thailand [
15–
17], India [
18], Iran [
19], and China [
20–
23], have reported 64 cases of infection with
B. mandrillaris, of which 32 were recently reported in 12 provinces in China. Patients infected by
B. mandrillaris can present symptoms of subacute to chronic cutaneous lesions or central nervous system (CNS) infection with granulomatous amebic encephalitis. In general, symptoms of this disease can be distinguished into 2 clinical manifestations. On the one hand, cases in the US tended to develop encephalitis without any concurrent skin lesions and usually died within a few months [
9]. The cases reported in China were less aggressive than those in the US, and were primarily characterized by the development of skin lesions. In addition, encephalitis occurred after several years, thereby increasing the chances of early successful treatment [
20]. However, the development of BAE rapidly deteriorates patients’ condition, with a fatality rate of over 95.0%, which can be explained by the delayed diagnosis and ineffective treatment approaches employed. Although the mortality rate of BAE is extremely high, its pathophysiology and pathogenesis remain unclear. Here we report on a female farmer from the Southwest China, who was infected by
B. mandrillaris manifested as BAE. Amebic pneumonia was finally diagnosed by the next-generation sequencing (NGS) technique. Unlike previous cases reported in China, the female patient of the present study directly developed encephalic infection without any symptoms of skin lesions, and then the patient’s condition deteriorated rapidly and died on day 22 after the onset of clinical symptoms.
Case Description
A previously healthy female farmer (64 years old) had a history of headache and abdominal pain on 3 days before the onset of this disease and presented with dizziness, nausea, vomiting, dry cough, and gait instability. No visual rotation, blurred vision, diarrhea and hematochezia, fever, coma, and convulsions were recognized. The patient was treated at Bin Chuan county hospital in Dali Bai Autonomous Prefecture, Yunnan province, but her symptoms were not improved. She was transferred to the No. 3 affiliated hospital of Dali University on June 16, 2020. Urinary incontinence occurred on admission.
Physical examination showed a temperature of 36.3°C, blood pressure of 129/91 mmHg, body weight of 45 kg, and symptoms of pain and weakness. She presented both bilateral horizontal and rotational nystagmus when looking to the right, and abduction of the right eye was limited. Despite the fact that the patient could successfully complete finger-nose, heel-knee-tibia, and hands alternating movement tests, Romberg’s sign was positive and she was unable to complete a straight walking test. Neck resistance was positive, while other meningeal signs were negative.
The results of the laboratory examination are shown in
Table 1. On day 9, the total white blood cells (WBC) number in the peripheral blood was normal, the proportion of neutrophils increased (78.7% NEUT), the proportion of lymphocytes decreased (14.9% LYM), and platelet increased (328×10
9/L PLT). Anti-TP (
Treponema pallidum) IgG antibody in serum was positive by ELISA testing, but negative by syphilis toluidine red untreated serum test (TRUST), which was consistent with the results obtained from the cerebrospinal fluid (CSF) on days 12 and 19 (
Table 2). Peripheral blood examination was repeated separately on days 11, 16, and 19 revealing that WBC (up to 11.2×10
9/L) was higher than normal, while the proportions of neutrophils and lymphocytes were increased (88.0% NEUT) and decreased (6.6% LYM), respectively. The erythrocyte sedimentation rate (ESR, 55 mm/h) on day 16 was elevated, and a 90-fold increase in the level of C-reactive protein (26.2 mg/L) was noted compared with day 11. CSF examination on day 12 showed the following: leukocytes/L: 24×10
6 (5.0% neutrophil, 95.0% lymphocyte); glucose: 2.64 mmol/L; CL: 112.2 mmol/L; and protein: 720.4 mg/L, which was following those repeated on day 19. CSF was clear and transparent, having a pressure of 145 mmHg, and was free from bacterial and fungal growth.
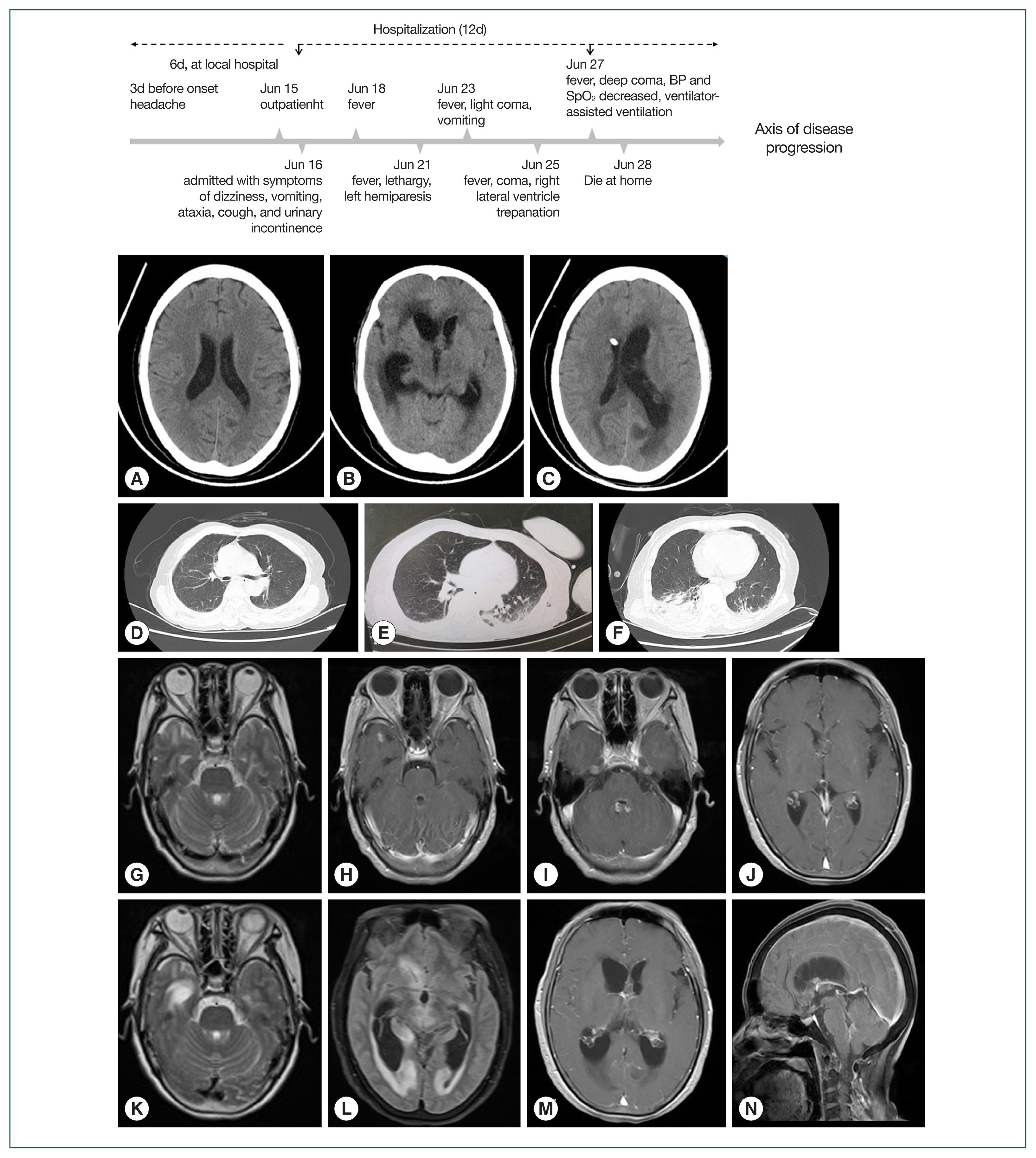
On day 9, craniocerebral computed tomography (CT) demonstrated multiple lacunar infarcts in the right basal ganglia (
Fig. 1). On day 11, a cranial contrast-enhanced magnetic resonance imaging (MRI) showed multiple nodular abnormal signals in the subependymal of the bilateral ventricles, third ventricle, fourth ventricle, right temporal lobe, and left occipital lobe.
Based on the clinical manifestations and laboratory data, the patient was primarily diagnosed with infectious meningitis and encephalitis and suspected syphilitic meningitis or neurotuberculosis. Intravenous ceftriaxone (2 g, q12h) was empirically administered on day 11. The patient developed fever (max 38.6°C) on day 12, and lethargy, language disorder, paralysis on the left limb, and neck stiffness on day 14. As a result ceftriaxone was discontinued, and her treatment was changed to penicillin (8 million units, q8h). However, the patient’s clinical condition deteriorated with worsening vomiting and superficial coma on day 15, and head MRI showed multiple patchy and low-density shadows beside the bilateral, third and fourth ventricles, further enlargement of bilateral ventricles, and obstructive hydrocephalus. On day 16, she was in a half comatose state and treated with dexamethasone (10 mg, QD), mannitol, and glycerol fructose to reduce intracranial pressure. However, the patient did not show any improvement (day 18). CSF drainage was then performed under local anesthesia, and the obtained results were consistent with previous findings, as shown in
Table 2. Additionally, her CSF sample was sent to the Kunming KingMed Diagnostics Group Co., Ltd for pathogen detection via Next Generation Sequencing (NGS). On day 19, she presented with symptoms of moderate coma, phlegm, and snoring, and sputum suction and tracheal intubation were then performed. A CT scan showed that the focus of intraventricular infection was the same as before, and CSF drainage from the right lateral ventricle was unobstructed. In addition, obstructive hydrocephalus of the left lateral ventricle was also noted, while the lymph nodes of the right lung were enlarged, and a large area of infection and a moderate amount of pleural effusion were observed, as shown in
Fig. 1. On day 21, she was in a deep coma with decreased oxygen saturation and placed on a ventilator. Given the significant drop in blood pressure, her family decided to cease the medical treatment, and she died at home the next day after discharge.
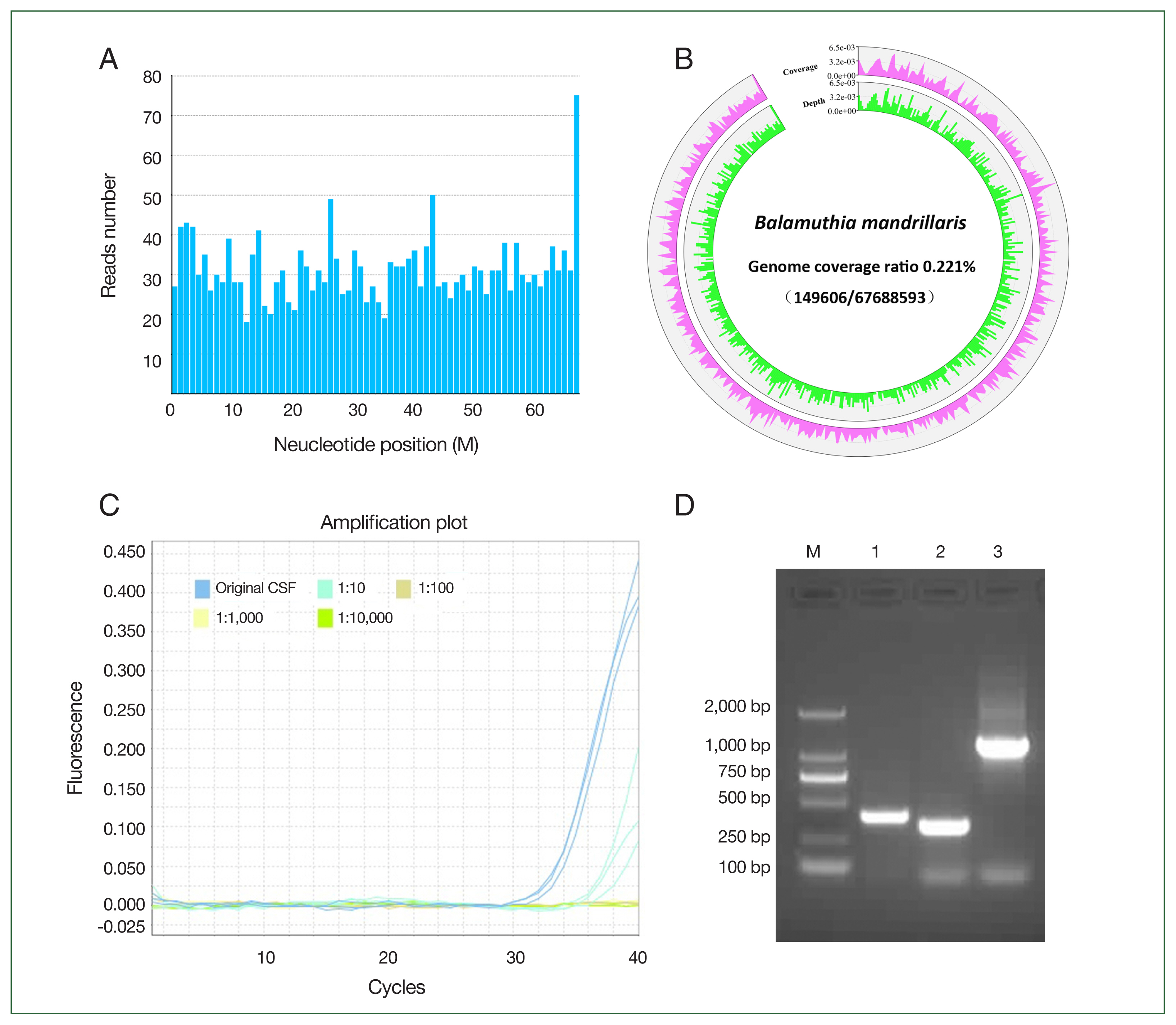
The NGS results showed that 2,136 reads of
B. mandrillaris were detected in the CSF (
Fig. S1). The mapped reads of
B. mandrillaris were evenly distributed across regions of the genome (reference genome GCA_001185145.1), and the sequencing coverage ratio was 0.2% (
Fig. 2A, B). Confirmation of
B. mandrillaris genomic DNA (QIAsymphony Circulating NA kit (Cus.48); QIAGEN, Dusseldorf, Germany) was conducted using PCR targeting at minimum 2 independent areas of the amebic genome. A
B. mandrillaris-specific region of the RNase P gene in the CSF was detected by TaqMan real-time PCR assay (ChamQ Universal SYBR qPCR Master Mix; Vazyme, Nanjing, China) using a primer set of RNP-F and RNP-R together with a TaqMan RNP probe with a Ct value of 33.27±0.36 (
Fig. 2C).
B. mandrillaris DNA was also amplified using specific primer sets, namely Balspec16S and Balspec16SR, BalaF1451 and BalaR1621, and Balspec16S and Balspec16SR2 (
Fig. 2D) (GenBank
rns accession numbers AF477012 to AF477018). The PCR products were used for sequencing after TA cloning and shared 99.0% homology with the
B. mandrillaris 16S RNA gene (
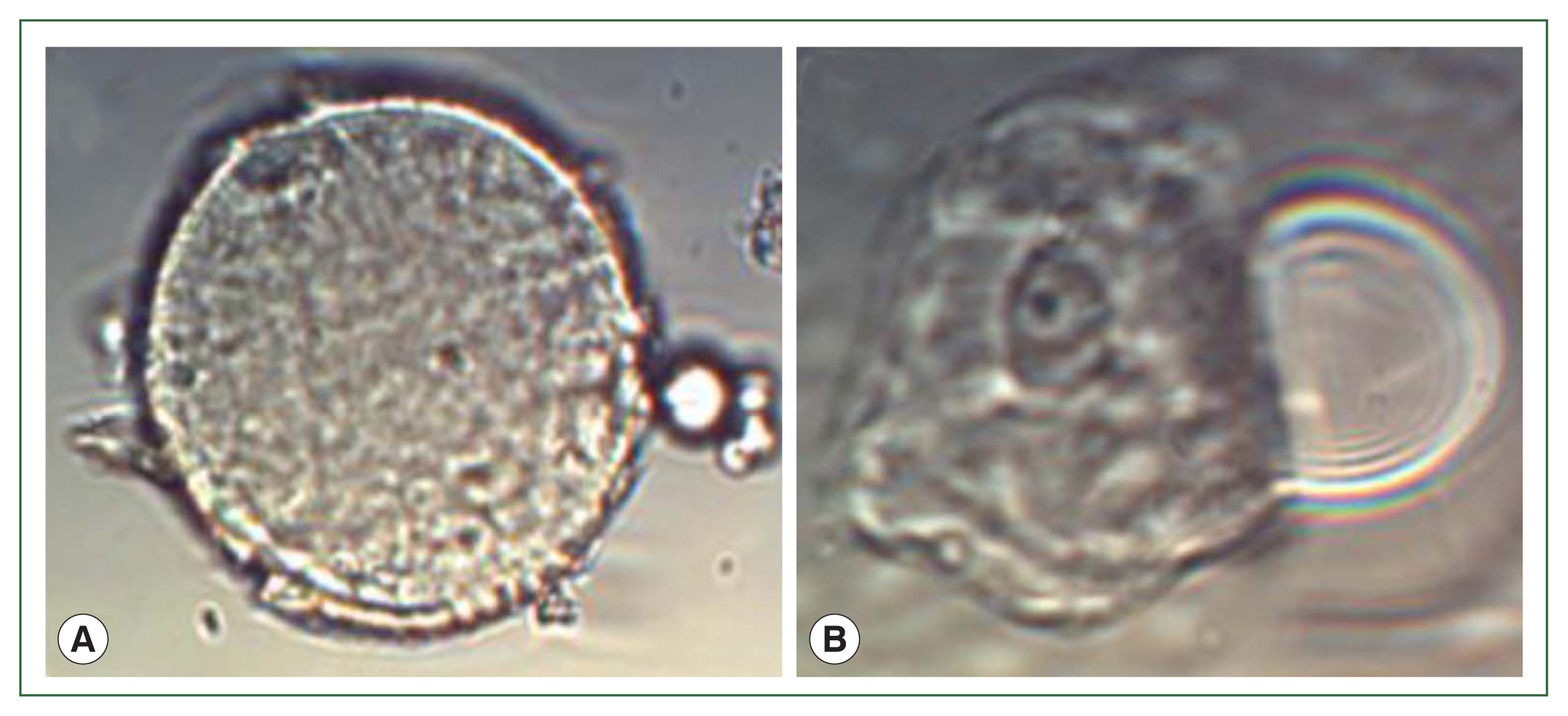
Fig. S2). The suspected cysts and trophozoites of
B. mandrillaris were examined in the sediment of patient’s CSF under microscope (
Fig. 3).
The patient was an elderly and frail farmer engaged in long-term field work in places where the climate is dry and hot. Her family denied her immunosuppression and medical history of consuming raw meat. According to the results of NGS [
22,
23], PCR detection [
24], and qRT-PCR targeting the RNase P gene [
25], combined with clinical symptoms and radiological findings, the patient was finally diagnosed with
B. mandrillaris amebic encephalitis and pneumonia.
Discussion
The life cycle of
B. mandrillaris is divided into 2 stages, namely trophozoites and cysts, and trophozoites enter the body through breathing or direct contact with the skin to cause infection [
10,
12]. This free-living ameba mainly affects the skin and CNS, but the clinical symptoms of CNS infection are untypical and can be easily confused with cerebral cysticercosis, abscess, cavity, tumor, and acute disseminated encephalomyelitis. Some patients occasionally develop pneumonia, bronchopneumonia, liver failure, renal failure, or severe multiple organ failure [
9]. Although the patient in the present study had a history of scratches on her left hand, she exhibited clinical symptoms of BAE without skin lesions but accompanied by pulmonary infection foci. Therefore, it was speculated that
B. mandrillaris trophozoites had invaded various organs through the respiratory tract, resulting in multiple infections. This case is quite different from the other 32 cases previously reported in China because
B. mandrillaris did not cause any skin lesions, but directly led to intracranial infection complicated with pneumonia. In addition, the course of the disease progressed so fast that the patient died in less than 3 weeks.
BAE may occur in immunocompetent or immunocompromised individuals. A previous survey showed that 39.0% of these cases suffer from diabetes, organ transplantation, HIV/AIDS, or use of immunosuppressants [
9,
10]. This may in turn increase the risk of BAE infection or accelerate disease progression, and also render the subsequent treatment efficacy poor (due to low immunity status). The incubation period is 1–7 days, the average age of onset is 36-years-old (ranging from 4 months to 91 years), and the average time from the onset of symptoms to death is 24 days (4–450 days). The average hospital stay is 21 days (4–372 days) [
9]. The patient in the present study was an elderly female, and the main clinical manifestations included symptoms of headache, dizziness, vomiting, cough, and ataxia. Urinary incontinence occurred on day 10. Fever began on the day 12 (the highest body temperature was 39.5°C), lethargy and mild coma appeared on day 15, and moderate coma occurred on day 16. Dexamethasone and mannitol were administered due to severe dehydration. Drilling and drainage of the right lateral ventricle were performed on day 19, and the patient underwent endotracheal intubation and ventilator-assisted breathing on day 20 as a result of deep coma and loss of spontaneous breathing. On day 21, the patient’s family decided to discontinue treatment, and the patient died at her home the next day after discharge. The time from the onset of clinical symptoms to death was only 22 days, and the average time of hospitalization was 12 days, rendering the progress of the disease faster than the average time reported in the literature [
2]. Unlike the previous cases reported in China, the patient showed a disease course that was similar to the cases presented in Europe and the United States.
Laboratory tests demonstrated that the patient’s peripheral blood leukocytes increased gradually after admission (up to 11.16×10
9), mainly neutrophils (88.0%), whereas lymphocytes decreased (min 6.6%), and hypokalemia, hyponatremia, and hypochloremia developed simultaneously. Corresponding to the peripheral blood analysis, the leukocytes in CSF increased (24×10
6), mainly lymphocytes (95.0% Lym), total protein increased, chlorine (CL) decreased, and glucose was either normal or decreased (
Table 1), results that are consistent with the findings reported in previous literature [
9]. Furthermore, previous research showed that the characteristic imaging finding of BAE was enhanced lesions (29.0%), multiple lesions (23.0%), or edema (27.0%) [
9]. The lesions were distributed throughout the brain area without specific localization. CT and MRI revealed the presence of multiple nodular abnormal signals in the bilateral, third, and fourth ventricles, subependymal, right temporal lobe, and left occipital lobe. Multiple swollen lymph nodes in the right lung, large foci of inflammatory infection, and pleural effusion were also noted (
Fig. 1). The erythrocyte sedimentation rate (55 mm/H) increased, and a 90-fold increase in the level of C-reactive protein (26.2 mg/L) was noted on day 7 compared with day 11 (
Table 1). Therefore, the above results indicate a preliminary diagnosis of CNS infection [
26].
The pathogens causing this infection were further analyzed in clinical samples. Neither acid-fast bacilli nor
Cryptococcus neoformans were observed in the centrifugal precipitation smear of CSF. Detection of
Mycobacterium tuberculosis (Mtb) by the Xpert MTB/RIF assay in CSF and Interferon-γ release assay (IGRA) in peripheral blood were negative, and latex agglutination test for the detection of capsular antigen of
Cryptococcus neoformans in CSF was also negative. These results indicated that neurotuberculosis and Cryptococcal meningitis had not been taken into consideration. In contrast, the anti-TP tests performed by ELISA and chemiluminescence assay (CMIA) in both peripheral blood and CSF were positive, and the reagin to TP detected by TRUST was negative in both peripheral blood and CSF (
Table 2), suggesting that the patient had been infected with TP. Considering the patient’s medical history, we concluded that this condition was transmitted from her husband due to his history of unsafe sexual intercourse. Therefore, she was mainly treated with empirical antibiotic therapy for neurosyphilis. However, the antisyphilis treatment employed deteriorated the patient’s condition. Antibodies of anti-HIV, HAV, HCV, and HBsAg antigen were negative in the patient’s serum, and her peripheral blood and CSF were also free of bacterial and fungal growth (
Table 2). In addition, antibodies to autoimmune encephalitis in the blood and CSF were negative. Hence, it was necessary to perform additional tests to identify that pathogen.
Due to the lack of characteristic clinical symptoms but also because the morphology of
B. mandrillaris is very similar to that of human tissue cells, BAE was difficulty to diagnose, [
20]. Epidemiological data showed that the average time from the onset of symptoms to the start of treatment was approximately 30 days (6–557 days), and 88.0% of BAE cases were diagnosed by brain biopsy, followed by indirect immunofluorescence (66.0%), PCR detection (47.0%), and histopathology examination (45.0%). In contrast, only 3.0% of BAE cases were diagnosed using conventional isolation and culture of pathogens. There are 2 main clinical manifestations of the
B. mandrillaris infection. First, BAE cases in China and Peru exhibited initial symptoms of skin lesions, usually a single lesion with occasional satellite lesions around it. Although, painless plaques on the face or extremities (especially knees) or ulcerated skin lesions could be cured by drugs, such as lincomycin and azithromycin, development of encephalitis after a few months or years would render treatment very difficult, increasing the respective mortality rates. In contrast, the cases reported in the US and Europe were characterized by the rapid development of encephalitis without symptoms of any skin lesions, causing patients’ death within a few months. Our case was very different from previous cases reported in China. The disease course progressed very rapidly, and the patient died in less than 3 weeks, which was similar to the cases reported in Europe and the US. Additionally, the cases reported in China and Peru developed skin lesions and were less invasive, allowing adequate time to diagnose this disease through skin biopsies, and provide effective medications and treatment. However, since it is very difficult to perform brain biopsies and autopsies due to the high influence of traditional concepts in China, PCR examination or NGS in CSF could only be conducted [
24,
27].
The incidence of
B. mandrillaris infections in humans is very rare. As such, early diagnosis, treatment, and prognosis of this condition are directly dependent on the physicians’ knowledge of the disease. NGS has been widely used in the diagnosis of rare diseases or new pathogenic infections, improving the sensitivity of sequencing-based diagnosis and monitoring [
23]. In the field of amebic encephalitis, NGS can be used to differentiate
Acanthamoeba spp.,
Naegleria fowleri, and
B. mandrillaris [
22]. More than 2000 DNA sequences uniformly distributed in the genome of
B. mandrillaris were found in the patient’s CSF by NGS sequencing, whereas no nucleic acid sequences of other pathogens, such as
Mycobacterium tuberculosis,
TP, and
Cryptococcus neoformans, were found. The RNase P gene and 16S RNA genes of
B. mandrillaris were further confirmed using TaqMan real-time PCR assay, PCR, and T-A cloning sequencing.
At present, there is no definite medication for treating BAE. Studies have shown that several drugs used alone or in combination according to their efficacy have a certain effect on the treatment of BAE, such as miltefosine [
21,
28], pentamidine, miconazole, 5-fluorocytosine, ketoconazole [
29], macrolides [
16], and nitroquinoline [
30]. The delayed diagnosis, misdiagnosis, and subsequent mistreatment of BAE may aggravate the disease, resulting in a global survival rate of less than 5.0%. In our treatment regime, the patient was treated with ceftriaxone and penicillin, while chemotherapy drugs were not used. Based on the clinical characteristics, epidemiological history, and molecular technique, the patient was diagnosed with
B. mandrillaris infection.
In summary, BAE is a fatal disease with low morbidity and high mortality rates due to the delayed diagnosis and ineffective treatment strategies employed, and children are especially prone to this rare infection. In our study, an elderly female with agriculture-related occupation was infected with B. mandrillaris, directly developed encephalitis, and died within 3 weeks of the onset of symptoms, which is substantially different from other cases reported in China. The results of the CSF examination showed elevated leukocytes with lymphocytes predominating, decreased chlorine (CL), and normal or decreased glucose. There was no bacterial and fungal growth in CSF. Neuroimaging examination showed multiple patchy lesions, and empirical treatment had no obvious effect or even worsened the course of the disease. The patient was finally diagnosed with BAE based on NGS and PCR techniques. This is the first case of BAE reported in southwestern China, which is of great significance in assisting physicians in the diagnosis and treatment of B. mandrillaris infection.
Notes
-
The authors declare no conflict of interest related to this study.
-
Author contributions
Conceptualization: Chen Z, Wu YC
Data curation: Chen X,
Formal analysis:, Wu YC
Funding acquisition: Wu YC
Investigation: Yao S
Methodology: Chen X, Qian L, Wu YC
Project administration: Wu YC
Resources: Wu YC
Software: Chen X
Supervision: Chen X, Wu Y
Validation: Wu YC
Visualization: Chen X,
Writing – original draft: Chen X, Wu YC
Writing – review & editing:, Wu C
Supplementary Information
Acknowledgments
We would like to thank the patient’s family for participating in the present research study. We would also like to thank Prof. Di Qu and Yang Wu from Fudan University for their helpful discussions, and Prof. Li Bai and Yuanying Shen from Dali University for the case analysis. This work was supported by the National Natural Science Foundation of China (No. 82060380, 81660346 to WYC), and by Young and the Middle-aged Academic Leader Training Foundation of Yunnan Province (No. 202305AC160038 to WYC).
All data generated or analyzed during this study were included in this published article and its
Supplementary Fig. S1.
Fig. 1Imageological features of a patient with Balamuthia mandrillaris disease. Computed tomography (CT) of the brain (A, B, and C) and the lung (D, E, and F) were performed on days 1, 9, and 13, respectively; magnetic resonance imaging (MRI) of the brain was initially performed on day 3 (G–J) and again on day 8 (K–N).
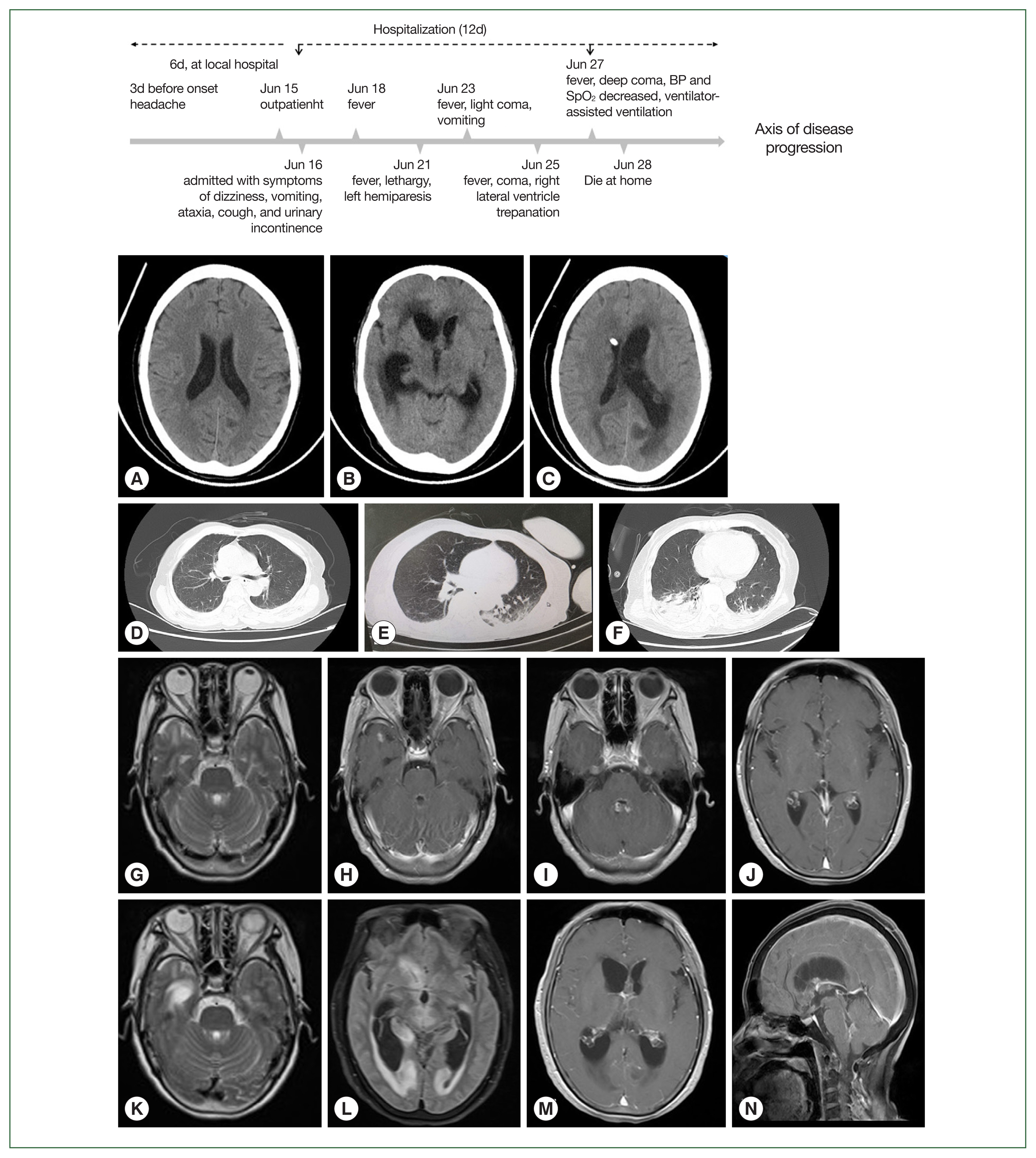
Fig. 2Identification of B. mandrillaris in the patient’s cerebrospinal fluid. (A) The presence of B. mandrillaris genomic DNA in the CSF sample was detected using next-generation sequencing (NGS), (B) sequencing coverage and depth, (C) TaqMan qRT-PCR targeting the RNase P gene, and (D) PCR amplification targeting of the 16S RNA gene (D). A: The mapped read number was distributed across the genomic region of B. mandrillaris. B: Whole genome of B. mandrillaris was divided into 500 equal parts, i.e., 500 window bins, and the coverage and depth of each bin were then calculated. Coverage represents the percentage covered by reads. Depth represents the average depth in the bin region. C: The CSF was 10-fold serially diluted, and qRT-PCR targeting the B. mandrillaris RNase P gene was performed using the primer set RNP-F, RNP-R, and TaqMan RNP probe. D: B. mandrillaris DNA was also amplified using primer sets, namely Balspec16S/Balspec16SR (lane 1, 395 bp), BalaF1451/BalaR1621 (lane 2, 336 bp), and Balspec16S/Balspec16SR2 (lane 3, 1, 240 bp). The PCR products were sequenced after T-A cloning.
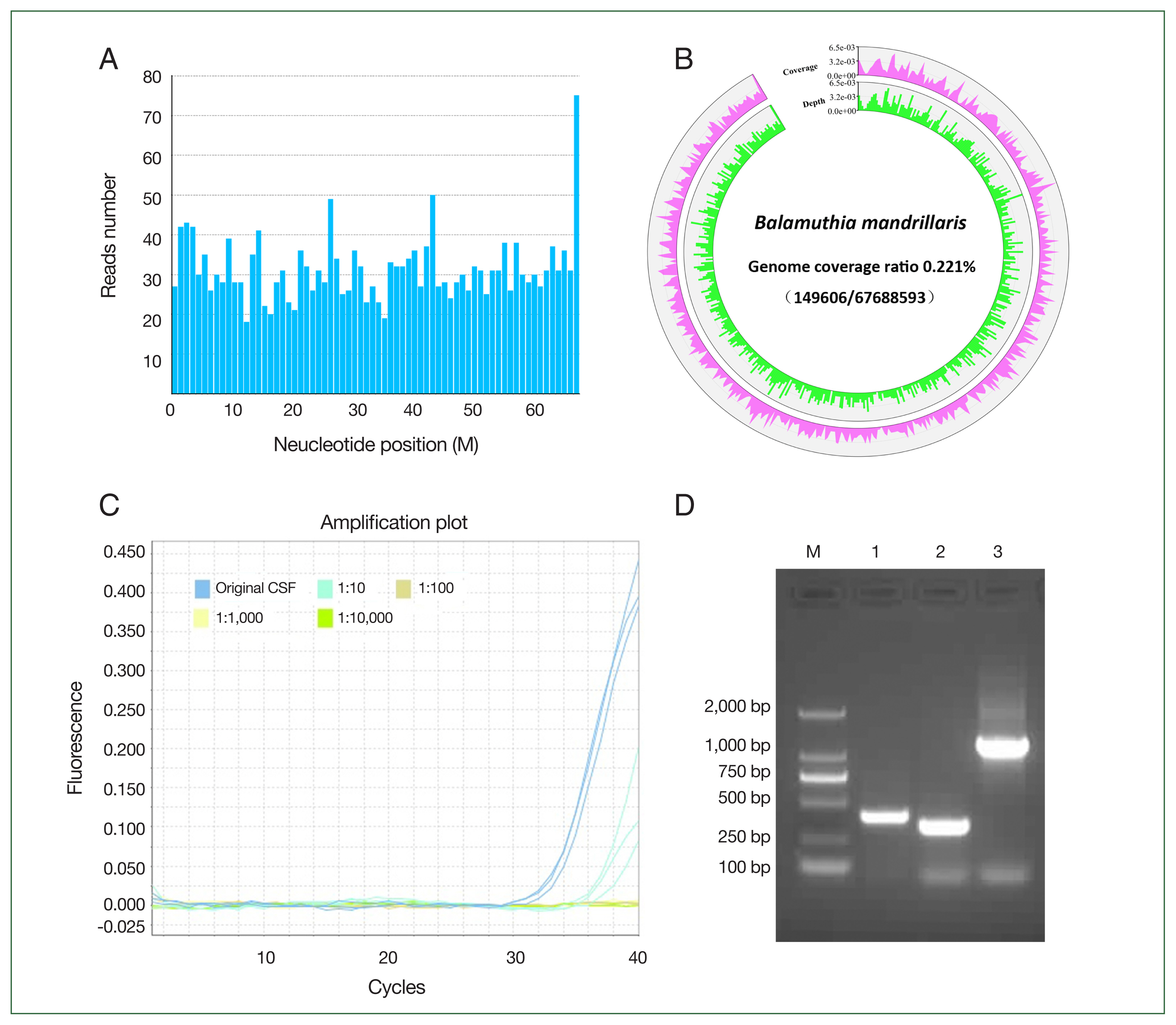
Fig. 3Microscopic image of B. mandrillaris in the patient’s cerebrospinal fluid. (A) Spherical cysts, measuring 15–20 μm in size; (B) Ovoid to round trophozoites, measuring 20–25 μm in size, and 1–2 nuclei containing 1–4 nucleoli were noted (Original magnification×40. The scale is 20 μm).
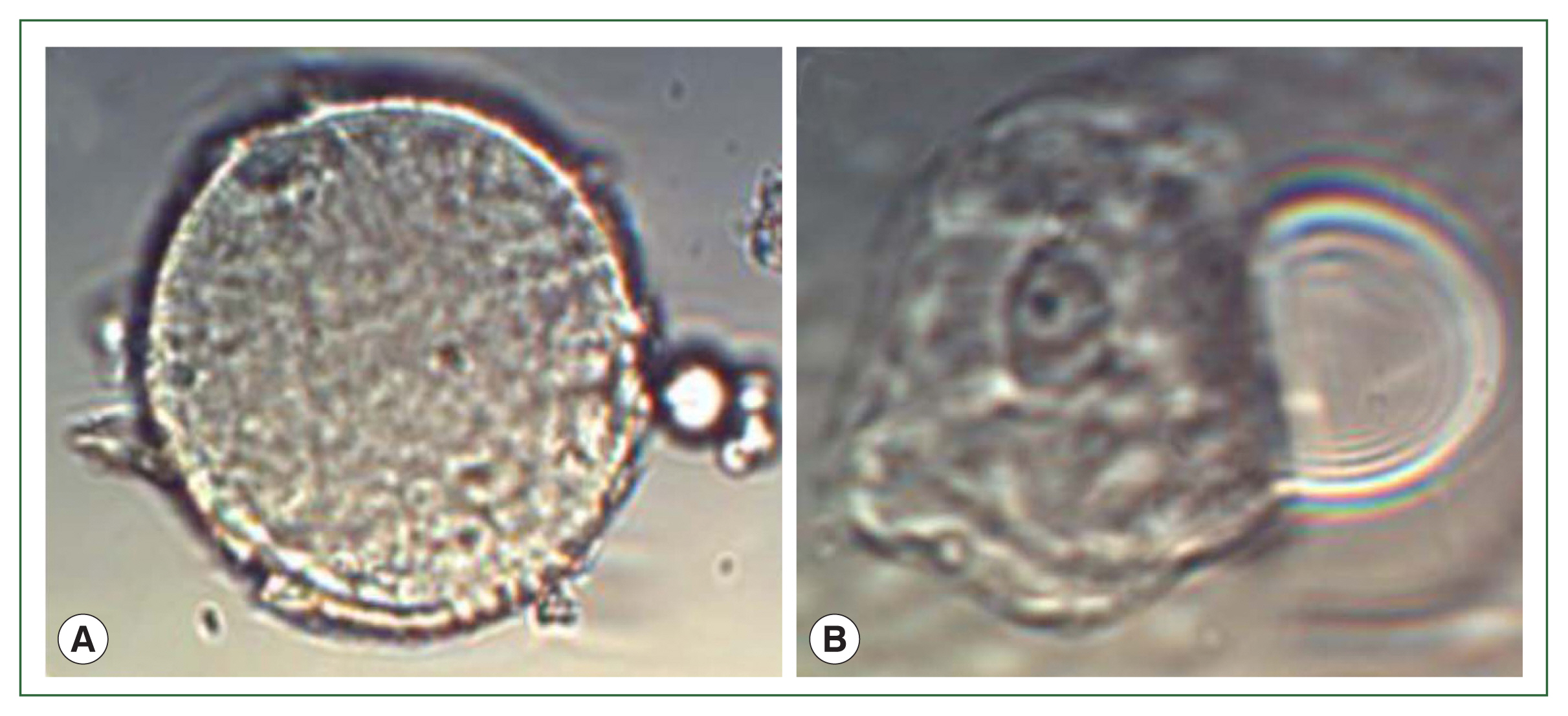
Table 1laboratory examination for the patient with Balamuthia mandrillaris encephalitis during the hospitalization
Table 1
|
Date |
Blood cells |
Electrolytes (mmol/L) |
GLU (mmol/L) |
Total Protein (g/L) |
ADA (U/L) |
ESR (mm/H) |
CRP (mg/L) |
|
|
|
WBC×109/L (Neu%, Lym%) |
RBC× 1012/L |
Platelet× 109/L |
Potassium |
Sodium |
Chloride |
Calcium |
Phosphate |
|
Peripheral blood |
|
Day 9 |
7.6 (78.7, 14.9) |
4.2 |
328 |
4.2 |
129.9 |
93.6 |
2.3 |
1.4 |
7.4 |
82.2 |
|
|
|
|
Day 11 |
8.0 (80.8, 11.7) |
4.0 |
339 |
3.2 |
126.8 |
89.8 |
2.1 |
1.2 |
8.4 |
71.8 |
|
|
0.3 |
|
Day 12 |
|
|
|
2.8 |
133.4 |
97.2 |
2.0 |
0.6 |
7.4 |
|
|
|
|
|
Day 14 |
|
|
|
3.7 |
132 |
92.7 |
2.1 |
0.8 |
|
|
|
|
|
|
Day 16 |
11.1 (88.0, 6.6) |
3.6 |
310 |
3.8 |
138.7 |
100.9 |
2.1 |
1.0 |
6.9 |
58.0 |
|
55 |
26.2 |
|
Day 19 |
8.3 (83.8, 10.3) |
4.1 |
406 |
4.3 |
143.0 |
98.9 |
2.2 |
1.4 |
7.9 |
70.9 |
|
|
|
|
Day 21 |
|
|
|
3.1 |
152.3 |
111.2 |
2.1 |
0.8 |
7.9 |
52.7 |
|
|
|
|
|
CSF |
|
Day 12 |
24×106/L (95.0% Lym) |
0–1/HP |
|
|
|
112.2 |
|
|
2.6 |
0.7 |
7.0 |
|
|
|
Day 19 |
9×106/L (less lymphocyte) |
UD |
|
|
|
113 |
|
|
3.0 |
0.6 |
10.3 |
|
|
Table 2Antigens, antibodies, smear, isolation and sequencing for the pathogens suspected in the Balamuthia mandrillaris infection
Table 2
|
Samples |
|
TB |
C. neoformans
|
Anti-TP |
Anti-HIV-1/2 |
Anti-HAV |
HBsAg |
Anti-HCV |
Culture |
NGS |
|
Day 9 |
Peripheral blood |
|
|
ELISA (IgG+)
TRUST (reagin−) |
ELISA − |
ELISA − |
ELISA − |
ELISA − |
|
|
|
Day 12 |
Peripheral blood |
|
|
|
|
|
|
|
No growth |
|
|
Day 12 |
CSF |
Acid-fast staining (−) |
Ink staining (−) |
ELISA (IgG+)
TRUST (reagin−)
CMIA (IgG+) |
|
|
|
|
No growth |
|
|
Day 16 |
Peripheral blood |
IGRA (−) |
|
|
|
|
|
|
No growth |
|
|
Day 19 |
CSF |
Xpert MTB/RIF (TB-DNA, UD) |
LAT (−) |
ELISA (IgG+)
TRUST (reagin−) |
|
|
|
|
No growth |
2,000 reads mapped the genome of BM |
References
- 1. Baquero RA, Reyes-Batlle M, Nicola GG, Martín-Navarro CM, López-Arencibia A, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health 2014;108(4):206-211. https://doi.org/10.1179/2047773214Y.0000000143
- 2. Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology (Reading) 2004;150(Pt 9):2837-2842. https://doi.org/10.1099/mic.0.27218-0
- 3. Niyyati M, Karamati SA, Lorenzo Morales J, Lasjerdi Z. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res 2016;115(2):541-545. https://doi.org/10.1007/s00436-015-4770-y
- 4. Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol 2003;41(7):3175-3180. https://doi.org/10.1128/JCM.41.7.3175-3180.2003
- 5. Retana-Moreira L, Abrahams-Sandí E, Cabello-Vílchez AM, Reyes-Batlle M, Valladares B, et al. Isolation and molecular characterization of Acanthamoeba and Balamuthia mandrillaris from combination shower units in Costa Rica. Parasitol Res 2014;113(11):4117-4122. https://doi.org/10.1007/s00436-014-4083-6
- 6. Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 1990;28(12):2750-2756. https://doi.org/10.1128/jcm.28.12.2750-2756.1990
- 7. Finnin PJ, Visvesvara GS, Campbell BE, Fry DR, Gasser RB. Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol Res 2007;100(2):423-426. https://doi.org/10.1007/s00436-006-0302-0
- 8. Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PH. Amebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxid ameba) in a horse. J Vet Diagn Invest 1998;10(4):378-381. https://doi.org/10.1177/104063879801000416
- 9. Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, et al. The Epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. Clin infect Dis 2019;68(11):1815-1822. https://doi.org/10.1093/cid/ciy813
- 10. Farnon EC, Kokko KE, Budge PJ, Mbaeyi C, Lutterloh EC, et al. Transmission of Balamuthia mandrillaris by organ transplantation. Clini Infect Dis 2016;63(7):878-888. https://doi.org/10.1093/cid/ciw422
- 11. Yamanouchi K, Arima H, Sakamoto Y, Kanto K, Kasai K, et al. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol Res 2018;117(9):2895-2900. https://doi.org/10.1007/s00436-018-5980-x
- 12. Kum SJ, Lee HW, Jung HR, Choe M, Kim SP. Amoebic Encephalitis Caused by Balamuthia mandrillaris. J Patho Transl Med 2019;53(5):327-331. https://doi.org/10.4132/jptm.2019.05.14
- 13. Lee JY, Yu IK, Kim SM, Kim JH, Kim HY. Fulminant disseminating fatal granulomatous amebic encephalitis: the first case report in an immunocompetent patient in South Korea. Yonsei Med J 2021;62(6):563-567. https://doi.org/10.3349/ymj.2021.62.6.563
- 14. Hara T, Yagita K, Sugita Y. Pathogenic free-living amoebic encephalitis in Japan. Neuropathology 2019;39(4):251-258. https://doi.org/10.1111/neup.12582
- 15. Intalapaporn P, Suankratay C, Shuangshoti S, Phantumchinda K, Keelawat S, et al. Balamuthia mandrillaris meningoencephalitis: the first case in southeast Asia. Am J Trop Med Hyg 2004;70(6):666-669.
- 16. Krasaelap A, Prechawit S, Chansaenroj J, Punyahotra P, Puthanakit T, et al. Fatal Balamuthia amebic encephalitis in a healthy child: a case report with review of survival cases. Korean J Parasitol 2013;51(3):335-341. https://doi.org/10.3347/kjp.2013.51.3.335
- 17. Sangruchi T, Martinez AJ, Visvesvara GS. Spontaneous granulomatous amebic encephalitis: report of four cases from Thailand. Southeast Asian J Trop Med Public Health 1994;25(2):309-313.
- 18. Khurana S, Hallur V, Goyal MK, Sehgal R, Radotra BD. Emergence of Balamuthia mandrillaris meningoencephalitis in India. Indian J Med Microbiol 2015;33(2):298-300. https://doi.org/10.4103/0255-0857.154887
- 19. Safavi M, Mehrtash V, Habibi Z, Mohammadpour M, Haghi Ashtiani MT, et al. Case report: encephalitis caused by Balamuthia mandrillaris in a 3-year-old iranian girl. Am J Trop Med Hyg 2021;104(5):1836-1840. https://doi.org/10.4269/ajtmh.20-1257
- 20. Wang L, Cheng W, Li B, Jian Z, Qi X, et al. Balamuthia mandrillaris infection in China: a retrospective report of 28 cases. Emerg Microbes Infect 2020;9(1):2348-2357. https://doi.org/10.1080/22221751.2020.1835447
- 21. Shehab KW, Aboul-Nasr K, Elliott SP. Balamuthia mandrillaris granulomatous amebic encephalitis with renal dissemination in a previously healthy child: case report and review of the pediatric literature. J Pediatric Infect Dis Soc 2018;7(3):e163-e168. https://doi.org/10.1093/jpids/pix089
- 22. Wu X, Yan G, Han S, Ye Y, Cheng X, et al. Diagnosing Balamuthia mandrillaris encephalitis via next-generation sequencing in a 13-year-old girl. Emerg Microbes Infect 2020;9(1):1379-1387. https://doi.org/10.1080/22221751.2020.1775130
- 23. Yang Y, Hu X, Min L, Dong X, Guan Y. Balamuthia mandrillaris-related primary amoebic encephalitis in China diagnosed by next generation sequencing and a review of the literature. Lab Med 2020;51(2):e20-e26. https://doi.org/10.1093/labmed/lmz079
- 24. Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol 2003;41(1):453-455. https://doi.org/10.1128/JCM.41.1.453-455.2003
- 25. Kiderlen AF, Radam E, Lewin A. Detection of Balamuthia mandrillaris DNA by real-time PCR targeting the RNase P gene. BMC Microbiol 2008;8:210. https://doi.org/10.1186/1471-2180-8-210
- 26. Mittal SO, Alsinaidi O. Teaching neuro Images: Balamuthia mandrillaris amebic encephalitis: clinical-radiologic-pathologic correlation. Neurology 2017;88(18):e183. https://doi.org/10.1212/WNL.0000000000003891
- 27. Booton GC, Schuster FL, Carmichael JR, Paul AF, Thomas JB. Balamuthia mandrillaris: identification of clinical and environmental isolates using genus-specific PCR. J Eukaryot Microbiol 2003;50(suppl):508-509. https://doi.org/10.1111/j.1550-7408.2003.tb00611.x
- 28. Yohannan B, Feldman M. Fatal Balamuthia mandrillaris encephalitis. Case Rep Infect Dis 2019;2019:9315756. https://doi.org/10.1155/2019/9315756
- 29. Siddiqui R, Matin A, Warhurst D, Stins M, Khan NA. Effect of antimicrobial compounds on Balamuthia mandrillaris encystment and human brain microvascular endothelial cell cytopathogenicity. Antimicrob Agents Chemother 2007;51(12):4471-4473. https://doi.org/10.1128/AAC.00373-07
- 30. Laurie MT, White CV, Retallack H, Wu W, Moser MS, et al. Functional assessment of 2,177 U.S. and international drugs identifies the quinoline nitroxoline as a potent amoebicidal agent against the pathogen Balamuthia mandrillaris. mBio 2018;9(5):e02051-18. https://doi.org/10.1128/mBio.02051-18
 , Xiaoting Chen1,†
, Xiaoting Chen1,† , Lian Qian1, Shizheng Sun1, Chunjing Zhao1, Zongkai Bai1, Zhaofang Chen1,*
, Lian Qian1, Shizheng Sun1, Chunjing Zhao1, Zongkai Bai1, Zhaofang Chen1,* , Youcong Wu1,3,*
, Youcong Wu1,3,*