Abstract
This study aimed to identify the recent risk factors for Opisthorchis viverrini infection and cholangiocarcinoma (CCA) to improve disease prevention. The participants were divided into the following 3 groups based on their health status: healthy control (nonOV and nonCCA), those with O. viverrini infection (OV), and those with CCA. A questionnaire was used to explore their lifestyle and behaviors. Multivariate logistic regression and backward elimination were used to identify the significant risk factors. The results showed that the significant risk factors for both O. viverrini infection and CCA were age>50 years (odd ratio (OR)=8.44, P<0.001, 95% confidence intervals (CI) 2.98–23.90 and OR=43.47, P=0.001, 95% CI 14.71–128.45, respectively) and raw fish consumption (OR=8.48, P< 0.001, 95% CI 3.18–22.63 and OR=3.15, P=0.048, 95% CI 1.01–9.86, respectively). A history of O. viverrini infection was identified as an additional risk factor for CCA (OR=20.93, P=0.011, 95% CI 2.04–215.10). This study provided an update on the risk factors for O. viverrini infection and CCA. Asymptomatic patients with O. viverrini infection, particularly those>50 years old, should be carefully monitored to prevent CCA.
-
Key words: Opisthorchiasis, cholangiocarcinoma, risk factor, old age
Cholangiocarcinoma (CCA) is a heterogeneous disease that refers to rare tumors originating from the bile duct epithelial cells. This is an uncommon cancer that has been reported to have a significant mortality rate [
1] and can arise in the intrahepatic, extrahepatic, and perihilar regions of the bile duct. The worldwide incidence of CCA has been increasing and is highest in the Lower Mekong region (i.e., Lao PDR, Cambodia, Vietnam, and Thailand), which is an endemic area for
Opisthorchis viverrini. In fact, the incidence of CCA in northeast Khon Kaen, Thailand was estimated to be 40 and 100 per 100,000 women and men, respectively [
2].
Infection with
O. viverrini, which is a carcinogenic liver fluke, is the leading risk factor for cholangiocarcinoma in Thailand, particularly in the northeast [
3]. This parasite was classified as a group 1 carcinogen by the International Agency for Research on Cancer. The prevalence of
O. viverrini infection in the area remains high, with rates surpassing 50% at the village level [
3,
4]. A previous epidemiological study has shown that the prevalence of
O. viverrini infection varied, with an average of 22.7%, among the provinces in the upper northeastern region of Thailand. Among these provinces, Nakhon Phanom has the highest rate of
O. viverrini infection at 40.9% [
5]. Nowadays, the combination of
O. viverrini infection with nitrosamine compounds is widely acknowledged as a substantial risk factor for cholangiocarcinoma [
6]. Furthermore, smoking and alcohol consumption [
7], as well as dietary habits and lifestyle, may be linked to the CCA tumorigenesis.
For almost a century, consumption of the traditional Thai cuisine of raw or undercooked fish (e.g., koi pla or chopped raw fish salad, pla som or briefly fermented fish, and pla ra or extensively fermented fish) has been recognized as a significant risk factor for
O. viverrini and CCA [
8,
9]. However, because of strong beliefs and misconceptions on raw or undercooked food, locals continue to consume undercooked fish unintentionally. For example, many locals believe that the acidity of the lime juice will cook the fish well, based on the change in its color, and kill the parasite. Others believe that concurrent consumption of alcoholic beverages can eliminate the parasite in raw food. In addition, some local people believe that food tastes better when raw or undercooked than when well-cooked. These beliefs and misconceptions are important risk factors of foodborne infection, including that with parasites [
10].
Therefore, this study aimed to determine the risk factors for O. viverrini infection and to present the recent updates on the habits of eating possible sources of O. viverrini metacercariae and nitrosamine-contaminated dishes. A set of questionnaires was used to investigate and identify the risk factors. Further analysis of the collected information will aid in identifying an asymptomatic patient infected with O. viverrini and may help prevent chronic infection and CCA development.
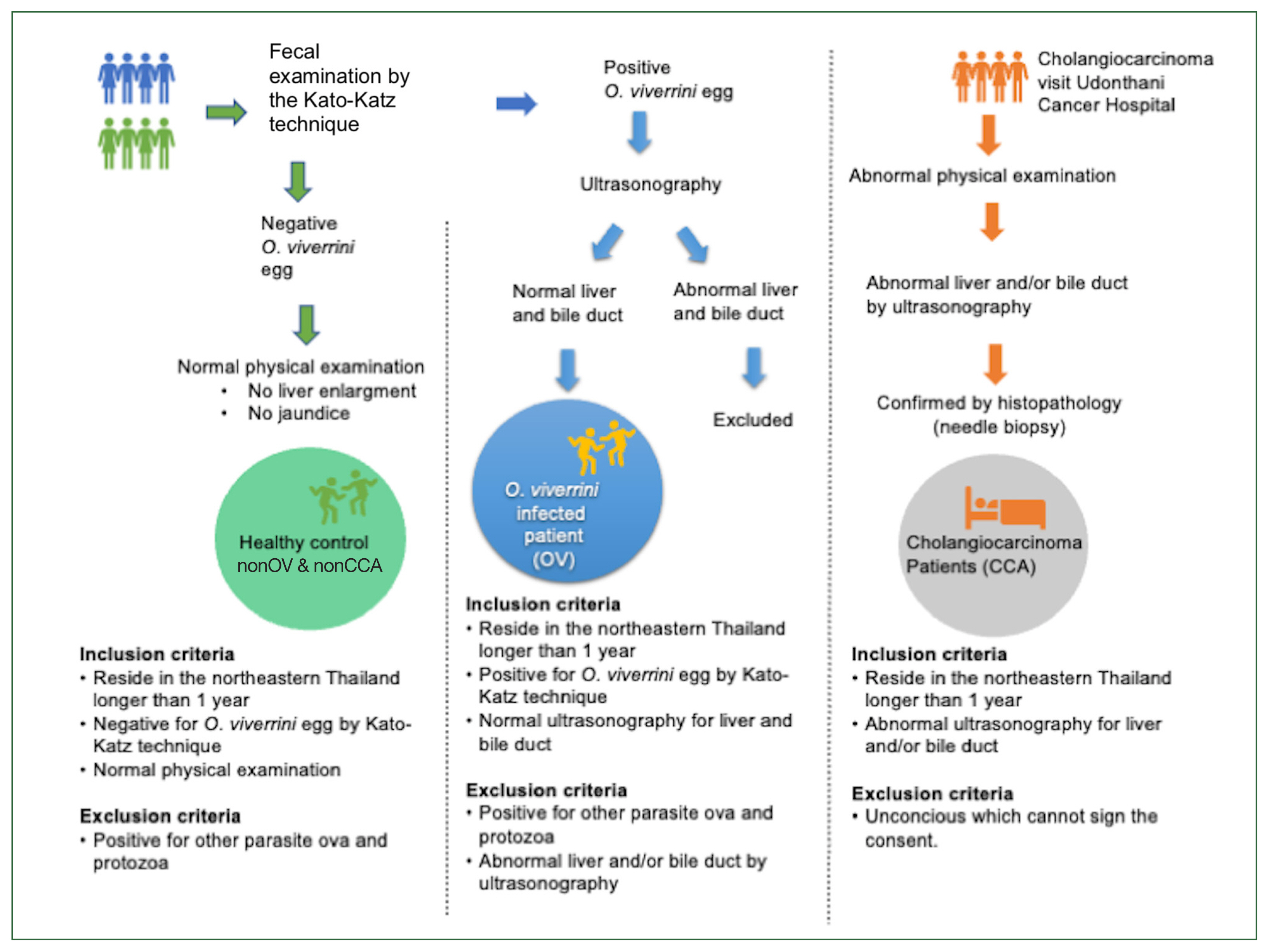
This study was conducted from September 2016 to September 2018 at Udon Thani and Nong Khai provinces. The questionnaires were retrieved and analyzed, which was approved by the Human Ethics Committee of Udonthani Cancer Hospital (UDCH-DPE 003/2021). The scheme of the recruitment process is summarized in
Fig. 1.
All enrolled subjects must have lived in northeastern Thailand for at least one year. The subjects were divided into 3 groups based on their health status, as follows: 1) healthy control group, for those who had no O. viverrini infection and CCA (nonOV and nonCCA); 2) OV, for those infected with O. viverrini; and 3) CCA, for those with CCA. The nonOV and nonCCA, and CCA groups were recruited from Udonthani Cancer Hospital, whereas the OV group was recruited from 4 health-promoting hospitals in the Nong Khai province, including the Nhong Luang, Kang Kai, Wat That, and Khok Chang, and Udon Thani provinces. A normal physical examination and a negative result for parasite eggs on the fecal examination were the recruitment criteria for the nonOV and nonCCA groups. For the OV group, 11, 89, and 1 subjects were enrolled from Udon Thani, Nong Khai, and Nakorn Phanom, respectively. The presence of fecal O. viverrini egg on the Kato–Katz method was defined as O. viverrini infection, based on the “The elimination of O. viverrini and cholangiocarcinoma” program of the Ministry of Public Health, Thailand. Under that program, at least 7,137 subjects from Udon Thani and Nong Khai were tested, and the results showed that 51 of 1,537 (3.3%) and 120 of 5,600 (2.1%) subjects, respectively, were positive for O. viverrini eggs. Among the subjects in the OV group, those who had normal liver and bile duct on ultrasound were invited to participate, whereas those who had abnormal ultrasound findings were excluded from this study. For CCA group, those who had histopathologically confirmed CCA were invited to participate.
The Kato–Katz concentration commercial kit was purchased from the Faculty of Tropi cal Medicine, Mahidol University, Bangkok, Thailand. The fecal samples were filtered through a sieve to remove debris then transferred to a 0.1-g template hole on a glass slide. Thereafter, a cellophane soaked overnight in malachite green glycerol solution was placed on top of the fecal sample. The glass slide was pressed to equally spread the feces on the cellophane. The parasite eggs were observed under light microscope after 20 min of incubation and were calculated as eggs per g.
All subjects were interviewed using a questionnaire, which aimed to identify the possible risk factors for O. viverrini-induced CCA (i.e., source of O. viverrini metacercaria and intake of nitrosamine-containing foods). To minimize misunderstanding, the questionnaire was filled in by a research assistant. In addition, the questionnaire included observations of lifestyle and behavior, such as smoking; alcohol consumption; habit of eating raw fish, which is the possible source of O. viverrini metacercaria; and fermented food-eating habit, which can be the source of nitrosamine. The suspected source of O. viverrini metacercariae was koi pla, which is a native Thai dish of raw chopped or minced fish mixed with shallot, lime juice, spring onion, and chili. The suspected as a source of nitrosamine was pla som, which is chopped, sliced, or minced Thai sour fish mixed with sticky or steamed rice and minced garlic, incubated at room temperature for 2–3 days, and fried before eating. Moreover, the questionnaire included intake of pla ra, which is raw fish mixed with sticky or steamed rice and minced garlic then incubated at room temperature for at least 6 months, and other fermented foods, such as fruits and vegetables. Furthermore, the questionnaire covered the history of O. viverrini infection, praziquantel treatment, underlying diseases, and medications.
The data were analyzed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY, USA). Based on a literature review, 10 variables were considered as potential predictors for the outcome analysis of
O. viverrini infection and CCA [
11,
12]. The association between the outcomes and their predictors was determined by multivariate logistic regression. All variables with
P<0.2 on the univariate analysis were used for the multivariate analysis. Backward elimination was used in the final model, with the same level of
P<0.2. The risk factors were considered significantly associated with the outcomes of
O. viverrini infection and CCA if
P value was <0.05. The adjusted odds ratios (OR) with their corresponding 95% confidence intervals (CI) were presented.
The characteristics of the participants are summarized in
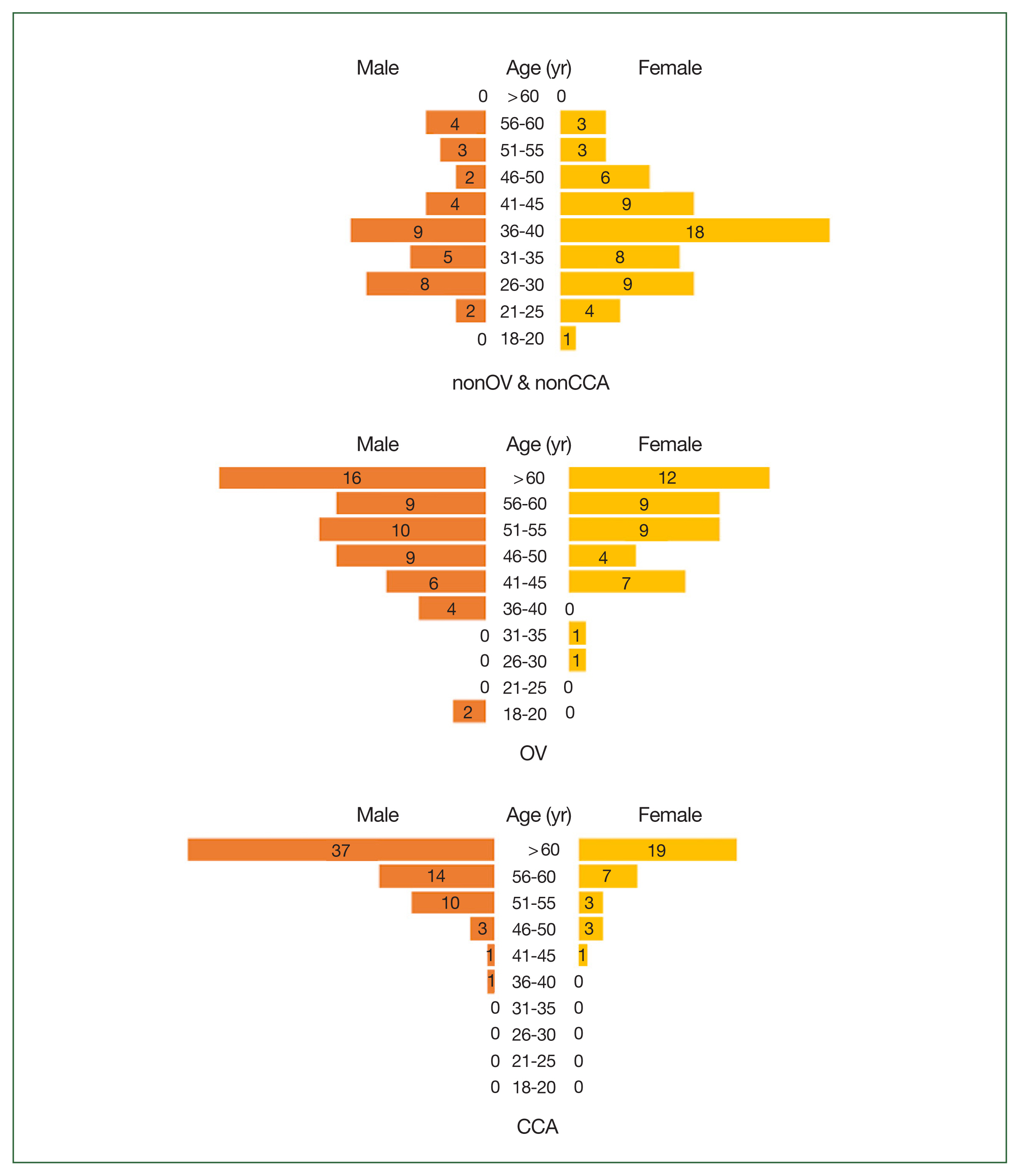
Table 1. The total number of participants was 98, 99, and 99, respectively. Compared with women, men had higher prevalence of OV (56.6%) and CCA (66.7%). The majority of the participants in the nonOV and nonCCA group were ≤50 years old (86.7%), whereas most of the participants in the OV and CCA groups were >50 years old (64.7% and 89.9%, respectively) (
Fig. 2). Alcohol consumption was similar between the nonOV and nonCCA and OV groups but was the highest in the CCA group (70.7%). Majority of the participants in the nonOV and nonCCA and OV groups did not smoke (83.7% and 64.7%, respectively); in the CCA group, the number of smokers and nonsmokers was almost equal. The rate of raw fish consumption was lowest in the nonOV and nonCCA group (33.7%) but was 84.9% in the OV and CCA groups. Dietary patterns of eating pla ra was common in all groups. Pla som consumption was the highest in the OV group (99.0%), followed by the CCA group (91.9%) and the nonOV and nonCCA group (76.5%). Majority of the participants in the OV and CCA groups did not consume other fermented foods (99.0% and 56.6%, respectively). A history of prior
O. viverrini infection was not found in 99.0% of the nonOV and nonCCA group and in 87.9% of the OV group but was noted in 30.3% of the CCA group.
The results of the multivariate logistic regression analysis of the risk factors for
O. viverrini infection are summarized in
Table 2. Sex was not a significant risk factor for
O. viverrini infection (
P=0.997), but age >50 years significantly increased the risk of infection (OR= 8.44,
P<0.001). Moreover, consumption of raw fish was shown to be a significant risk factor, whereas consumption of other fermented foods had a negative association with
O. viverrini infection (OR=0.01,
P<0.001). Compared with the participants who did not consume raw fish, those who did had 8.48 times more likelihood of being infected with
O. viverrini (
P<0.001). Among all the risk factors contributing to
O. viverrini infection, the risk factor that had the highest impact was raw fish consumption, followed by age >50 years.
As shown in
Table 3, multivariate logistic regression analysis identified age, raw fish con sumption, other fermented food consumption, and
O. viverrini infection history as the significant risk factors for CCA. Age>50 years increased the risk of CCA (OR=43.47,
P=0.001). Compared with the participants who did not consume raw fish, those who did were 3.15 times more likely to have CCA (
P=0.048). Furthermore, a history of
O. viverrini infection was found to have a significant role in CCA (OR=20.93,
P=0.011). On the other hand, sex (
P=0.498), alcohol consumption (
P=0.077), and pla som consumption (
P=0.067) were not significant risk factors. Moreover, consumption of other fermented food had a negative association with CCA (OR=0.34,
P=0.045).
The primary source of
O. viverrini infection is freshwater fish [
11], including fermented fish, such as pla ra and pla som. However, in this study, it was not determined to be a leading risk factor for
O. viverrini infection. Contrary to this study, a previous research indicated that consumption of fermented fish was one of the main risk factors for
O. viverrini infection [
13]. On the other hand, consumption of raw fish, such as koi pla, was identified as a leading risk factor for
O. viverrini infection and CCA, consistent with the results of several previous studies [
12,
14–
16]. People living in the countryside, particularly in the northeast area, have a traditional culture of consuming raw fish. According to a recent study, distance from the river was one factor that influenced the decision to consume raw fish [
17]. Furthermore, locals prefer to cook simple meals, such as koi pla, which can be made with readily available ingredients, such as herbs, lime juice, or ant eggs.
Aside from raw fish consumption, age >50 years was a significant risk factor for
O. viverrini infection and CCA in our study. This may be accounted for by the fact that compared with younger individuals, older individuals have more frequent chances of eating raw or undercooked fish. Moreover, the habit of eating raw or undercooked fish might be less common in the younger generation. However, these results contradicted the findings of Prakobwong et al. (2017), who suggested that the rate of
O. viverrini infection decreased in individuals over the age of 50 years [
18]. On the other hand, various studies provided similar results of an increasing rate of
O. viverrini infection with age [
15].
Cholangiocarcinoma arises from chronic infection with O. viverrini. This study demonstrated a history of O. viverrini infection as the main risk factor for CCA. Furthermore, participants who had a history of O. viverrini infection were likely to be reinfected, probably because their habit of raw fish consumption remained unchanged. These may explain the significant role of a history of O. viverrini infection in the development of CCA.
Based on the questionnaire, all participants who were infected with O. viverrini were asymptomatic. Therefore, the annual health check-up program effectively identified asymptomatic patients. Consequently, use of the significant risk factors that were demonstrated in this study is highly recommended to identify high risk groups for O. viverrini infection and CCA. These high risk groups can be advised to participate in a health check program for disease detection. As mentioned earlier, inclusion of tests for O. viverrini infection and CCA in the national health program is recommended in areas that have a high incidence of these diseases in order to ultimate improve disease prevention.
In this study, sampling bias may have occurred because of the differences in the selected collection sites, particularly in light of the low incidence of O. viverrini infection. Nevertheless, it is important to highlight that all subjects resided in the upper part of northeastern Thailand and shared similar nationality, race, eating habits, lifestyle, education, climate, occupation, environment, and exposure to risk factors.
Notes
-
Author contributions
Conceptualization: Prasopdee S, Butthongkomvong K, Tesana S, Thitapakorn V
Data curation: Prasopdee S, Rojthongpond T, Chitkoolsamphan Y, Pholhelm M, Yusuk S, Butthongkomvong K, Kulsantiwong J, Phanaksri T, Kunjantarachot A, Tesana S, Sathavornmanee T, Thitapakorn V
Formal analysis: Prasopdee S, Rojthongpond T, Chitkoolsamphan Y, Pholhelm M, Yusuk S, Pattaraarchachai J, Butthongkomvong K, Phanaksri T, Tesana S, Thitapakorn V
Funding acquisition: Prasopdee S, Thitapakorn V
Investigation: Prasopdee S, Butthongkomvong K, Kulsantiwong J, Phanaksri T, Kunjantarachot A, Thitapakorn V
Methodology: Prasopdee S, Butthongkomvong K, Kulsantiwong J, Phanaksri T, Kunjantarachot A, Tesana S, Thitapakorn V
Project administration: Prasopdee S, Thitapakorn V
Resources: Prasopdee S
Software: Prasopdee S, Pattaraarchachai J
Writing – original draft: Prasopdee S, Rojthongpond T, Chitkoolsamphan Y, Pholhelm M, Yusuk S, Pattaraarchachai J, Butthongkomvong K, Thitapakorn V
Writing – review & editing: Pholhelm M, Yusuk S, Phanaksri T, Kunjantarachot A, Tesana S, Sathavornmanee T, Thitapakorn V
-
The authors declare no conflict of interest related to this study.
Acknowledgements
The authors thank all the patients who participated in the study and the Chulabhorn International College of Medicine (CICM) for supporting the laboratory and facilities. We would like to express our gratitude to Dr. Isara Chiawiriyabunya, who is the Director of Udonthani Cancer Hospital for the guidance and for supporting the laboratory and facilities.
This study was financially supported by the Thai Government Research Fund through Thammasat University (Fund Contract No. 50/2559 and 64/2560) and was partially supported by the Thammasat University Research Unit on Opisthorchiasis, Cholangiocarcinoma, and Neglected Parasitic Diseases to V. Thitapakorn and S. Prasopdee, and the Thai Government Research Fund through Thammasat University (Fund Contract No. 36/2562) to S.Prasopdee.
Fig. 1Procedure of subject recruitment. Physical examination, fecal examination, and ultrasound are used to categorize the subjects.
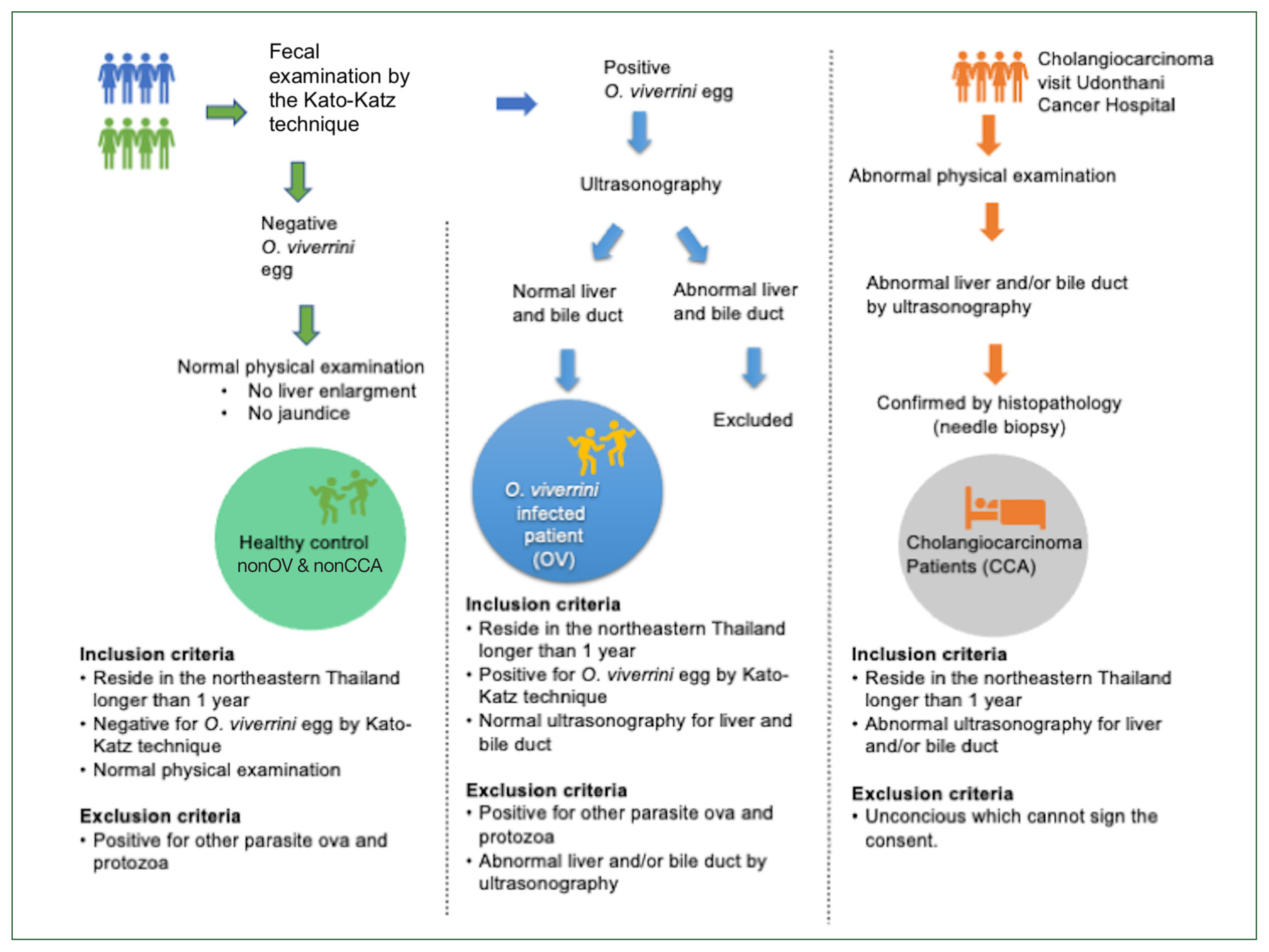
Fig. 2Age and sex distribution of the study population. The healthy control subjects (nonOV and nonCCA) (upper), patients with O. viverrini infection (OV) (middle), and patients with cholangiocarcinoma (lower) are shown.
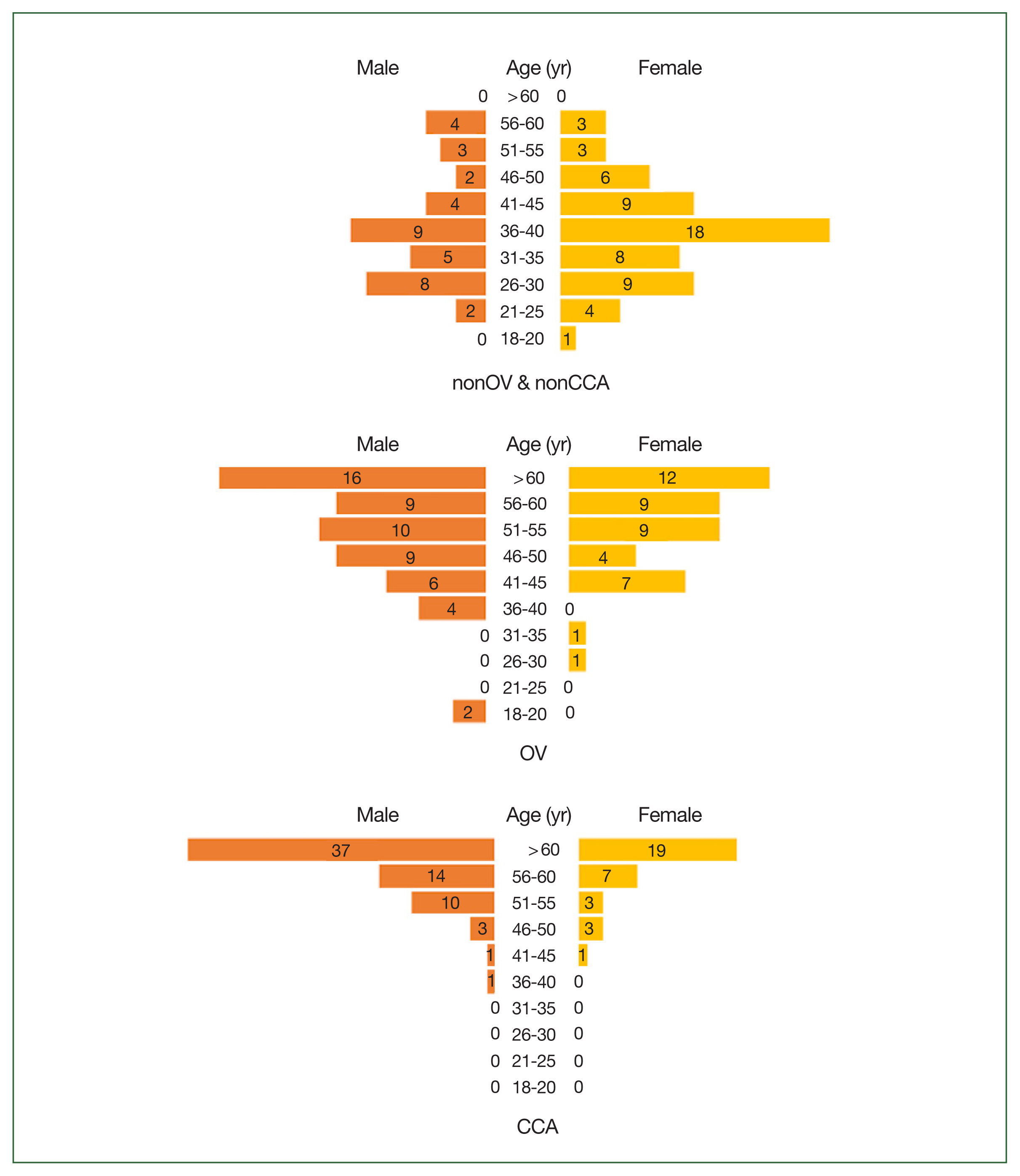
Table 1Characteristics of the participants
Table 1
|
Characteristic |
Category |
No. (%) |
|
|
nonOV & nonCCA |
OV |
CCA |
|
Subject number |
|
98 (100) |
99 (100) |
99 (100) |
|
|
Sex |
Female |
62 (63.2) |
43 (43.4) |
33 (33.3) |
|
Male |
36 (36.8) |
56 (56.6) |
66 (66.7) |
|
|
Age (years) |
≤50 |
85 (86.7) |
35 (35.3) |
10 (10.1) |
|
>50 |
13 (13.3) |
64 (64.7) |
89 (89.9) |
|
|
Alcohol consumption |
No |
51 (52.0) |
50 (50.5) |
29 (29.3) |
|
Yes |
47 (48.0) |
49 (49.5) |
70 (70.7) |
|
|
Smoking |
No |
82 (83.7) |
64 (64.7) |
47 (47.5) |
|
Yes |
16 (16.3) |
35 (35.4) |
52 (52.5) |
|
|
Raw fish consumption (Koi pla) |
No |
65 (66.3) |
15 (15.2) |
15 (15.2) |
|
Yes |
33 (33.7) |
84 (84.9) |
84 (84.9) |
|
|
Pla ra consumption |
No |
5 (5.1) |
0 (0.0) |
4 (4.0) |
|
Yes |
93 (94.9) |
99 (100) |
95 (96.0) |
|
|
Pla som consumption |
No |
23 (23.5) |
1 (1.0) |
8 (8.1) |
|
Yes |
75 (76.5) |
98 (99.0) |
91 (91.9) |
|
|
Other fermented foods consumption |
No |
35 (35.7) |
98 (99.0) |
56 (56.6) |
|
Yes |
63 (64.3) |
1 (1.0) |
43 (43.4) |
|
|
History of O. viverrini infection |
No |
97 (99.0) |
87 (87.9) |
69 (69.7) |
|
Yes |
1 (1.0) |
12 (12.1) |
30 (30.3) |
|
|
History of taking praziquantel |
No |
82 (83.7) |
87 (87.9) |
76 (76.8) |
|
Yes |
16 (16.3) |
12 (12.1) |
23 (23.2) |
|
|
Cancer stage |
I |
- |
- |
2 (0.0) |
|
II |
- |
- |
5 (0.1) |
|
III |
- |
- |
2 (0.0) |
|
IV |
- |
- |
90 (90.9) |
Table 2A multivariate logistic regression analysis of risk factors for O. viverrini infection
Table 2
|
Rick factor |
|
N (%) |
Adjusted OR |
95% CI |
P-value |
|
Sex |
Female |
105 (53.30) |
1.00 |
- |
- |
|
Male |
92 (46.70) |
1.00 |
0.39–2.60 |
0.997 |
|
|
Age (years) |
≤50 |
120 (60.91) |
1.00 |
- |
- |
|
>50 |
77 (39.09) |
8.44 |
2.98–23.90 |
<0.001 |
|
|
Raw fish consumption |
No |
80 (40.61) |
1.00 |
- |
- |
|
Yes |
117 (59.39) |
8.48 |
3.18–22.63 |
<0.001 |
|
|
Other fermented foods consumption |
No |
133 (67.51) |
1.00 |
- |
- |
|
Yes |
64 (32.49) |
0.01 |
0.00a-0.06 |
<0.001 |
Table 3A multivariate logistic regression analysis of risk factors for cholangiocarcinoma
Table 3
|
Rick factor |
|
N (%) |
Adjusted OR |
95% CI |
P-value |
|
Sex |
Female |
95 (48.22) |
1.00 |
- |
- |
|
Male |
102 (51.78) |
0.65 |
0.19–2.24 |
0.498 |
|
|
Age (years) |
≤50 |
95 (48.22) |
1.00 |
- |
- |
|
>50 |
102 (51.78) |
43.47 |
14.71–128.45 |
<0.001 |
|
|
Alcohol consumption |
No |
80 (40.61) |
1.00 |
- |
- |
|
Yes |
117 (59.39) |
3.24 |
0.88–11.90 |
0.077 |
|
|
Raw fish consumption |
No |
80 (40.61) |
1.00 |
- |
- |
|
Yes |
117 (59.39) |
3.15 |
1.01–9.86 |
0.048 |
|
|
Pla som consumption |
No |
31 (15.74) |
1.00 |
- |
- |
|
Yes |
166 (84.26) |
4.06 |
0.91–18.15 |
0.067 |
|
|
Other fermented foods consumption |
No |
91 (46.19) |
1.00 |
- |
- |
|
Yes |
106 (53.81) |
0.34 |
0.12–0.98 |
0.045 |
|
|
History of O. viverrini infection |
No |
166 (84.26) |
1.00 |
- |
- |
|
Yes |
31 (15.74) |
20.93 |
2.04–215.10 |
0.011 |
References
- 1. Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, et al. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 2011;120(suppl):158-168.
https://doi.org/10.1016/j.actatropica.2010.07.006
- 2. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, et al. Cholangiocarcinoma. Nat Rev Dis Primers 2021;7(1):65.
https://doi.org/10.1038/s41572-021-00300-2
- 3. Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Odermatt Sinuon M, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 2012;61(1):10-16.
https://doi.org/10.1016/j.parint.2011.08.014
- 4. Sripa B, Tangkawattana S, Laha T, Kaewkes S, Mallory FF, et al. Toward integrated opisthorchiasis control in northeast Thailand: the Lawa project. Acta Trop 2015;141(Pt B):361-367.
https://doi.org/10.1016/j.actatropica.2014.07.017
- 5. Thaewnongiew K, Singthong S, Kutchamart S, Tangsawad S, Promthet S, et al. Prevalence and risk factors for Opisthorchis viverrini infections in upper Northeast Thailand. Asian Pac J Cancer Prev 2014;15(16):6609-6612.
https://doi.org/10.7314/apjcp.2014.15.16.6609
- 6. Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res 1978;38(12):4634-4639.
- 7. Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci 2010;101(3):579-585.
https://doi.org/10.1111/j.1349-7006.2009.01458.x
- 8. Prasongwatana J, Laummaunwai P, Boonmars T, Pinlaor S. Viable metacercariae of Opisthorchis viverrini in northeastern Thai cyprinid fish dishes--as part of a rational program for control of O. viverrini-associated cholangiocarcinoma. Parasitol Res 2013;112(3):1323-1327.
https://doi.org/10.1007/s00436-012-3154-9
- 9. Sriraj P, Boonmars T, Aukkanimart R, Songsri J, Sripan P, et al. A combination of liver fluke infection and traditional northeastern Thai foods associated with cholangiocarcinoma development. Parasitol Res 2016;115(10):3843-3852.
https://doi.org/10.1007/s00436-016-5148-5
- 10. Sriraj P, Aukkanimart R, Boonmars T, Wonkchalee N, Juasook A, et al. Alcohol and alkalosis enhance excystation of Opisthorchis viverrini metacercariae. Parasitol Res 2013;112(6):2397-2402.
https://doi.org/10.1007/s00436-013-3346-y
- 11. Pengput A, Schwartz DG. Risk factors for Opisthorchis viverrini infection: a systematic review. J Infect Public Health 2020;13(9):1265-1273.
https://doi.org/10.1016/j.jiph.2020.05.028
- 12. Songserm N, Promthet S, Sithithaworn P, Pientong C, Ekalaksananan T, et al. Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol 2012;36(2):89-94.
https://doi.org/10.1016/j.canep.2011.11.007
- 13. Jadsri S, Noojoy A. A study of liver fluke infection in Sukhothai, Thailand. Southeast Asian J Trop Med Public Health 1999;30(3):588-593.
- 14. Manwong M, Songserm N, Promthet S, Matsuo K. Risk factors for cholangiocarcinoma in the lower part of Northeast Thailand: a hospital-based case-control study. Asian Pac J Cancer Prev 2013;14(10):5953-5956.
https://doi.org/10.7314/apjcp.2013.14.10.5953
- 15. Nakbun S, Thongkrajai P, Nithikathkul C. Risk factors for Opisthorchis viverrini infection in Nakhon Phanom, Thailand, where the infection is highly endemic. Asian Biomedicine 2018;12(1):45-51.
https://doi.org/10.1515/abm-2018-0030
- 16. Rangsin R, Mungthin M, Taamasri P, Mongklon S, Aimpun P, et al. Incidence and risk factors of Opisthorchis viverrini infections in a rural community in Thailand. Am J Trop Med Hyg 2009;81(1):152-155.
- 17. Ong X, Wang YC, Sithithaworn P, Namsanor J, Taylor D, et al. Uncovering the pathogenic landscape of helminth (Opisthorchis viverrini) infections: a cross-sectional study on contributions of physical and social environment and healthcare interventions. PLoS Negl Trop Dis 2016;10(12):e0005175.
https://doi.org/10.1371/journal.pntd.0005175
- 18. Prakobwong S, Suwannatrai A, Sancomerang A, Chaipibool S, Siriwechtumrong N. A large scale study of the epidemiology and risk factors for the carcinogenic liver fluke Opisthorchis viverrini in Udon Thani Province, Thailand. Asian Pac J Cancer Prev 2017;18(10):2853-2860.
https://doi.org/10.22034/APJCP.2017.18.10.2853
 , Thittinan Rojthongpond2
, Thittinan Rojthongpond2 , Yanwadee Chitkoolsamphan2
, Yanwadee Chitkoolsamphan2 , Montinee Pholhelm1,2
, Montinee Pholhelm1,2 , Siraphatsorn Yusuk1,2
, Siraphatsorn Yusuk1,2 , Junya Pattaraarchachai2
, Junya Pattaraarchachai2 , Kritiya Butthongkomvong3
, Kritiya Butthongkomvong3 , Jutharat Kulsantiwong4
, Jutharat Kulsantiwong4 , Teva Phanaksri2
, Teva Phanaksri2 , Anthicha Kunjantarachot2
, Anthicha Kunjantarachot2 , Smarn Tesana1
, Smarn Tesana1 , Thanakrit Sathavornmanee5
, Thanakrit Sathavornmanee5 , Veerachai Thitapakorn1,2,*
, Veerachai Thitapakorn1,2,*