Evaluation of the antimalarial activity of SAM13-2HCl with morpholine amide (SKM13 derivative) against antimalarial drug-resistant Plasmodium falciparum and Plasmodium berghei infected ICR mice
Article information
Abstract
Antimalarial drugs are an urgently need and crucial tool in the campaign against malaria, which can threaten public health. In this study, we examined the cytotoxicity of the 9 antimalarial compounds chemically synthesized using SKM13-2HCl. Except for SKM13-2HCl, the 5 newly synthesized compounds had a 50% cytotoxic concentration (CC50) >100 μM, indicating that they would be less cytotoxic than SKM13-2HCl. Among the 5 compounds, only SAM13-2HCl outperformed SKM13-2HCl for antimalarial activity, showing a 3- and 1.3-fold greater selective index (SI) (CC50/IC50) than SKM13-2HCl in vitro against both chloroquine-sensitive (3D7) and chloroquine -resistant (K1) Plasmodium falciparum strains, respectively. Thus, the presence of morpholine amide may help to effectively suppress human-infectious P. falciparum parasites. However, the antimalarial activity of SAM13-2HCl was inferior to that of the SKM13-2HCl template compound in the P. berghei NK65-infected mouse model, possibly because SAM13-2HCl had a lower polarity and less efficient pharmacokinetics than SKM13-2HCl. SAM13-2HCl was more toxic in the rodent model. Consequently, SAM13-2HCl containing morpholine was selected from screening a combination of pharmacologically significant structures as being the most effective in vitro against human-infectious P. falciparum but was less efficient in vivo in a P. berghei-infected animal model when compared with SKM13-2HCl. Therefore, SAM13-2HCl containing morpholine could be considered a promising compound to treat chloroquine-resistant P. falciparum infections, although further optimization is crucial to maintain antimalarial activity while reducing toxicity in animals.
Introduction
Malaria is an acute febrile illness transmitted by female Anopheles mosquitoes that are infected with protozoan parasites of the genus Plasmodium. In 2021, the World Health Organization reported that almost half of the global population was at risk of malaria infection caused by 5 parasite species (Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi); P. falciparum and P. vivax are the most transmitted and prevalent species [1].
Artemisinin-based combination therapy is a recognized treatment for the most prevalent malarial parasite, P. falciparum, and rapidly and comprehensively eliminates the parasites to prevent the progression of an uncomplicated case of malaria to severe disease or death [1]. However, drug resistance compromises the efficiency of malaria treatment and elimination. The emergence of antimalarial drug resistance is well documented among malaria parasites, with artemisinin resistance identified in the Greater Mekong Subregion and several African areas (Eritrea, Rwanda, and Uganda) [2,3]. The first artemisinin-resistant P. falciparum was reported in western Cambodia and has since spread to Southeast Asia [3–5]. Consequently, the discovery of new antimalarial drugs is urgently required to address the public health threat presented by malaria.
We previously reported that SKM13 and its derivatives exhibit antimalarial activity against human malaria in vitro and rodent malaria in vivo [6–8], although the efficacy of these antimalarial compounds did not surpass that of chloroquine (CQ). However, the low antimalarial efficacy of SKM13 derivatives compared with that of CQ in animal studies prompted the development of various derivatives with improved efficacy.
In our previous study, the SKM13 template was identified as an effective antimalarial candidate drug; however, intravenous (i.v.) administration of a high-concentration solution of SKM13 in dimethyl sulfoxide (DMSO) could induce discomfort in mice [6]. Typically, salt screening is implemented early in the drug research process to improve the developability and maximize in vivo exposure in orally administered solid dosage forms [9]. For parenteral products, increased solubility can help prevent precipitation at injection [10]. Therefore, to increase the solubility of SKM13, the salt form of SKM13 with HCl was developed; however, low plasma concentrations were detected, indicating poor efficiency attributed to limited intestinal adsorption [7]. This indicated that further research was required to counterbalance the hydrophobicity and hydrophilicity of SKM13 derivatives. Therefore, we modified the amide group of SKM13, rather than HCl, to maintain the balance between adsorption and hydrophilicity.
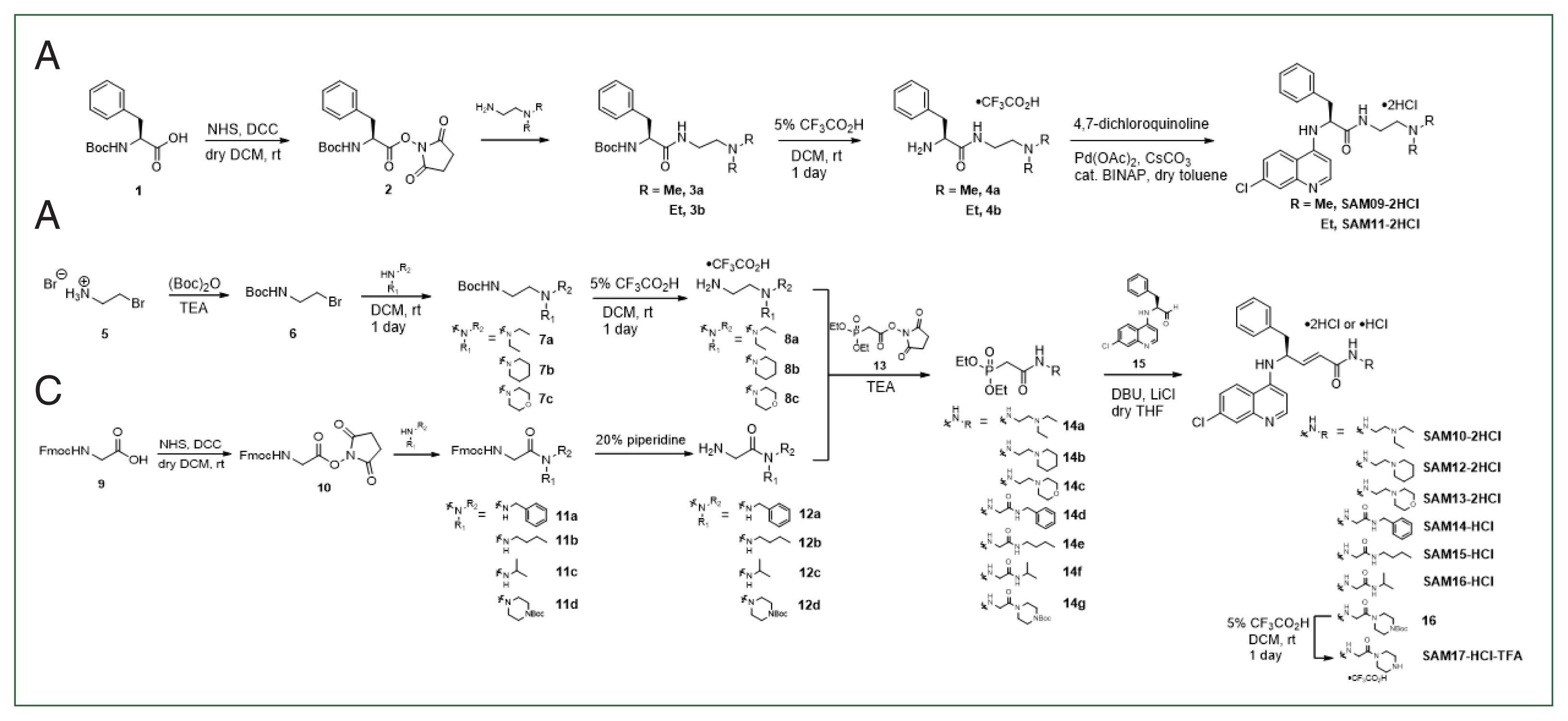
We synthesized 9 novel derivatives from the SAM series based on the structure of the SKM13 template. We synthesized the SAM series primarily by varying the side chain length, replacing the ethylenediamine moiety with a glycine unit, or linking the ends of the side chains with rings to alter the overall conformation and polarity. We examined the biological activities of the synthesized compounds, including their in vitro and in vivo toxicity and antimalarial efficacy. The solubility and pharmacokinetics (PK) in mice were measured to clarify the effect of inducing a single structural modification of the studied compounds on the balance between their hydrophobicity and hydrophilicity.
Materials and Methods
Reagents
Chloroquine, sodium bicarbonate, and hypoxanthine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gentamicin, SYTOX green solution, AlbuMAX I Lipid-Rich bovine serum albumin, and RPMI 1640 medium supplemented with 25 mM HEPES and L-glutamine were purchased from Gibco (Life Technologies Corporation, Grand Island, NY, USA). The Differential Quick III Stain Kit was purchased from Polysciences Inc. (Valley Road, Warrington, PA, USA). CQ-sensitive strain P. falciparum (3D7) (American Type Culture Collection, ATCC PRA-405D) and Vero cells (CRL-1587) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). A multiple antimalarial drug-resistant strain, P. falciparum K1 (MRA-159), and a CQ-susceptible strain, P. berghei NK65 (MRA-268), were obtained from BEI Resources (Manassas, VA, USA) [11].
Antimalarial drug synthesis
All derivatives (SAM09–17) were synthesized as previously reported with several improvements, such as the Horner–Wadsworth–Emmons reaction [11]. Synthetic schemes and chemical structures are summarized in the Methods section of the Supplementary Information, including the proton nuclear magnetic resonance spectra.
Determination of the UV absorbance
The UV absorbances of SKM13-2HCl and SAM13-2HCl were measured using a Jasco V-750 model UV/Vis spectrometer, with each sample prepared in a mixed solvent of water/AcCN/MeOH (6/3/1). The absorbance intensities of the respective samples, measured at 342 nm, were 0.817906 and 0.61802.
Determination of the solubility of SKM13-2HCl and SAM13-2HCl standards in water
The solubility of SAM13-2HCl and SKM13-2HCl was determined using ultrahigh-performance liquid chromatography (Thermo Fisher Scientific). Retention times for SKM13-2HCl and SAM13-2HCl were 7.35 and 8.14 min, respectively. The UV length was set at λmax=342 nm. Linear regression analysis was used to determine the aqueous solubility of SKM13-2HCl and SAM13-2HCl.
Cytotoxicity test using the MTT assay
The 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay was performed as previously reported [6]. Briefly, Vero cells were seeded in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 104 cells per well in a 96-well cell culture plate. Twelve hours after seeding, serial concentrations of each compound were added and incubated for 2 days at 37°C and 5% CO2. Subsequently, 20 μl of MTT reagent was added to each well, followed by incubation at 37°C and 5% CO2 for 2 h. The absorbance was measured at 490 nm using a microplate reader (Tecan Group Ltd., Canton of Zurich, Switzerland). The percentage of growth inhibition was calculated based on the 50% cytotoxic concentration (CC50).
Culture of malaria and antimalarial activity test in vitro
The blood stage of the P. falciparum 3D7 and K1 strains was propagated in 5% hematocrit of O+ human red blood cells suspended in optimal media [7]. Giemsa staining of methanol-fixed thin blood smears was used to monitor parasite growth [8]. Synchronized parasites were used to measure the antimalarial activity of each compound, which was determined using the half-maximal inhibitory concentration of malarial growth (IC50) [7].
In vivo suppression of rodent malaria (4-day suppressive test)
A 4-day suppressive test was performed to determine the in vivo antimalarial activity of the promising SAM candidate [6]. Briefly, 6-week-old ICR-1 mice were inoculated with 107 blood-stage rodent malaria strain P. berghei NK65 on day 0. Subsequently, mice were intravenously administered with drug treatment at different concentrations (10, 20, and 30 mg/kg) once daily for 4 days, and the body weight, survival, and blood parasites were assessed daily for 2 weeks. Eight days postinfection (dpi), mice were euthanized, and mouse livers and spleens were harvested.
In vivo PK assessment in mice
Healthy ICR female and 7-week-old mice (approximately 30 g) were used to analyze PK properties. ICR mice were provided by Orient Bio (Gyeonggi-do, Republic of Korea) and maintained at an institutional animal facility under specific pathogen-free conditions at 18–23°C and 40–60% humidity during the experimental period. All procedures involving animals followed the Regulations of the Experimental Animal Administration issued by Seoul National University (IACUC # SNU-220502-10-1). Three mice were orally administered with the drug solution at 5 mg/kg in a single dose (100 μl/mouse); blood was then collected at different times, and the plasma obtained was stored at −80°C. The plasma concentration of each compound was plotted to generate a regression trend line to establish the kinetics of each compound using PK Solver 2.0 as previously described [7,12].
Statistical analysis
Statistical analysis was performed using a 2-way analysis of variance and Bonferroni post hoc test with GraphPad (version 9.0; GraphPad Software, San Diego, California, USA). All experiments were performed in triplicate, and data were expressed as the mean±SD.
Results
Antimalarial drug synthesis and quantification
Nine new derivatives were synthesized (SAM09-2HCl to SAM17-2HCL) based on the previously published skeleton of SKM13-2HCl [7] (Fig. 1). The side chain derived from phenylalanine was modified to synthesize these novel derivatives via 3 strategies. The structural modifications and features are summarized as follows: 1) The first strategy (SAM09-2HCl and SAM11-2HCl) shortened the length of the side chain by removing the double bond of SKM13-2HCl and was expected to increase water solubility; 2) The second strategy (SAM10-2HCl, SAM12-2HCl, and SAM13-2HCl) modified the N, N-dimethylamine amide at the end of the SKM13-2HCl side chain to a heterocycle amide or N, N-diethylamine amide; this extended the existing structure or introduced a ring structure to interfere with the free rotation and should cause a distinct interaction with the target protein; 3) The third strategy (SAM14-HCl, SAM15-HCl, SAM16-HCl, and SAM17-HCl-TFA) replaced the ethylene diamine in the side chain of SKM13-2HCl with a glycine unit to alter the aqueous solubility, along with the addition of extra hydrogen bonds.
In vitro cytotoxicity and antimalarial activity
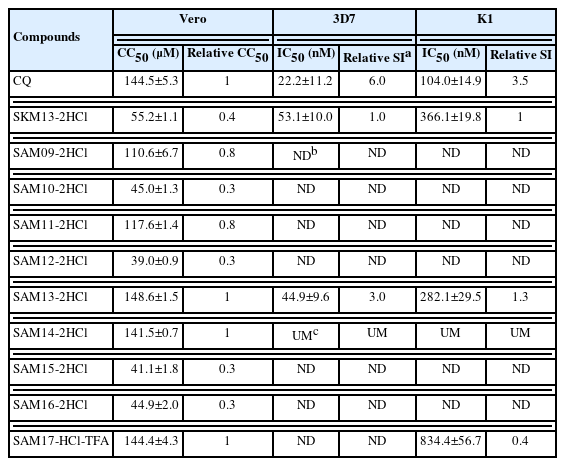
During the development of any novel antipathogen compound, the cytotoxic concentration of the compound that can kill 50% of viable cells in the host (CC50) must be evaluated. The 9 structurally diverse compounds were therefore assessed for cytotoxicity in Vero cells in the MTT assay following a 2-day compound exposure. Five SAMs (09, 11, 13, 14, and 17) of the 9 derivatives had a CC50>50 μM while the remaining 4 compounds had CC50 values <50 μM (Table 1).
Three candidates (SAM13-2HCl containing morpholine, SAM14-2HCl, and SAM17-HCl-TFA with a glycine unit) were less cytotoxic than the SKM13-2HCl template with comparable cytotoxicity to that of CQ. These candidates were selected for further assessment of antimalarial activity in vitro with the 3D7 and K1 strains (CQ-sensitive and resistant P. falciparum, respectively).
The half-maximal inhibitory concentration (IC50) of SAM13-2HCl was 44.9±9.6 and 282.1±29.5 nM against 3D7 (CQ-sensitive) and K1 (CQ-resistant), respectively, indicating that SAM13-2HCl exhibited better antimalarial activity than SKM13-2HCl, which had IC50 values of 53.1±10.0 and 366.1±19.8 nM against 3D7 and K1, respectively (Table 1). SAM14-2HCl did not suppress either parasite, whereas SAM17-HCl-TFA exerted a marginal effect on K1. The final SI (CC50/IC50) of SAM13-2HCl was 3- and 1.3-fold higher than that of SKM13-2HCl in 3D7 and K1, respectively, indicating that SAM13-2HCl could efficiently suppress human-infectious P. falciparum parasites.
Curative effects of antimalarial compounds in a rodent model
We previously examined the antimalarial activities of the new derivatives in an animal model using the rodent P. berghei NK65 strain [6]. SAM13-2HCl exhibited a better SI value than SKM13-2HCl, and the relative difference between the 2 compounds was investigated in a rodent model using P. berghei (a CQ-susceptible strain) as previously described [7]. Accordingly, a 4-day suppression test was performed in mice using 10, 20, and 30 mg/kg of SAM13-2HC1 and SKM13-2HCl (single daily administration). The inhibitory effects of i.v. administration of SKM13-2HCl or SAM13-2HCl on parasitemia and body weight in vivo are shown in Fig. 2.

Parasitemia and body weight in P. berghei NK65–infected mice (n=5) after treatment with antimalarial compounds in a 4-day suppression test. ***P<0.001. Closed red circle, parasitemia; open square, body weight.
The daily increase in parasitemia was significantly increased by up to 10% in the infected-untreated group from 3 dpi (Fig. 2). Treatment with CQ at 10 mg/kg (i.v.) significantly reduced parasite density throughout the study. Treatment with 10 mg/kg SKM13-2HCl increased parasitemia, whereas 20 and 30 mg/kg SKM13-2HCl effectively suppressed parasitemia, indicating that the suppressive effect of SKM13-2HCl on parasitemia was less effective at 10 mg/kg, which was the CQ concentration.
SAM13-2HCl increased parasitemia when compared that in the group treated with SKM13-2HCl at 10 mg/kg (P<0.001), indicating that SAM13-2HCl was less effective than SKM13-2HCl in suppressing parasitemia in rodent infectious P. berghei–infected mice (Fig. 2). The body weight of SAM13-2HCl (10 mg/kg)–treated mice started to decrease at 6 days, although this was not observed in SKM13-2HCl–treated mice. These findings correspond with the increased parasitemia observed with SAM13-2HCl treatment.
Curative effect of antimalarial compounds on the survival of P. berghei NK65–infected rodents after a single daily administration
No mortality was evident at 6 dpi in the P. berghei–infected group while parasitemia was increased by up to 10%, which corresponded to results in a previous study [7]. Thus, 6 dpi was sufficient time to compare the efficacy of SKM13-2HCl and SAM13-2HCl. Although 20 mg/kg SAM13-2HCl treatment had a 60% survival rate, this decreased to 40% with 30 mg/kg SAM13-2HCl, indicating the potential toxic effect of SAM13-2HCl. Conversely, the highest concentration of SKM13-2HCl (30 mg/kg) had a complete curative effect in animals.
Hemozoin is phagocytosed by macrophages in the liver and spleen, where heme crystals can persist for months to years in Plasmodium-infected animals [13]. Therefore, liver pigmentation is a good indicator of antimalarial drug efficacy. The P. berghei–infected group showed an enlarged spleen and dark-colored liver with pigmentation, indicating infection. However, SKM13-2HCl at 20 mg/kg decreased liver pigmentation. In addition, the spleens of SKM13-2HCl (20 mg/kg)–treated mice were the same size as those of the normal or CQ-treated groups; liver pigmentation and spleen enlargement persisted with 30 mg/kg SAM13-2HCl treatment (Fig. 3), corroborating the poor efficacy of SAM13-2HCl compared with that of SKM13-2HCl in a P. berghei–infected rodent model.
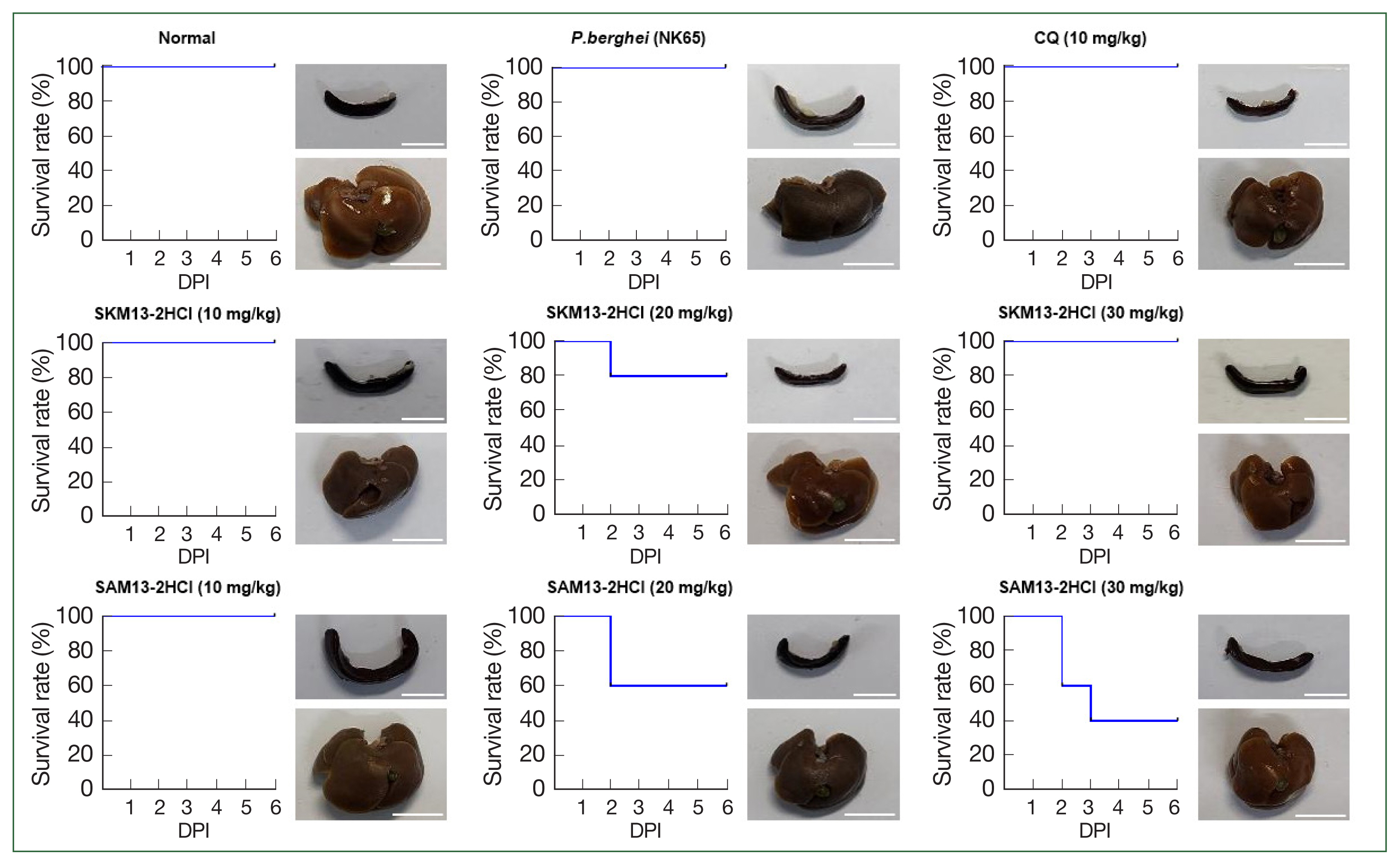
Curative effects of antimalarial compounds against P. berghei NK65 in a 4-day suppression test. The survival rate of P. berghei NK65–infected mice (n=5) was observed at 6 dpi after treatment once daily. At 8 dpi, the livers and spleens of euthanized mice were collected. DPI, days postinfection. Scale bar=1 cm.
PK of the compounds
The excretion rates and steady-state concentrations of a specific drug are determined by its half-life [14] and were determined via LC–MS/MS in this study; calibration and quality control standards were analyzed in the study sample batches. Cmax is the maximum serum concentration of a drug in a specific test area of the body after administration [7] and reflects the rate and extent of absorption. Following oral administration, SKM13-2HCl and SAM13-2HCl exhibited Cmax values of 515±0.04 and 52±0.3 μM, respectively, indicating that the serum concentration of SKM13-2HCl was higher than that of SAM13 at peak concentration.
Cmax is highly correlated with the area under the curve (AUC) and is used to compare blood concentrations over time. Therefore, the Cmax/AUC ratio is recommended to assess the equivalence of absorption rates [15]. The Cmax/AUC of SKM13-2HCl was 2-fold higher than that of SAM13-2HCl, and the adsorption rate of SKM13-2HCl may therefore be better than that of SAM13-2HCl. This corresponded to Tmax_h, the time to peak concentration in hours, which was 4-fold lower with SKM13-2HCl (0.25 h) than with SAM13-2HCl (1 h). However, the elimination half-lives (t1/2) of both drugs were similar. The raw results of the PK measurements are presented in Supplementary Fig. S1.
Solubility of the compounds in deionized water
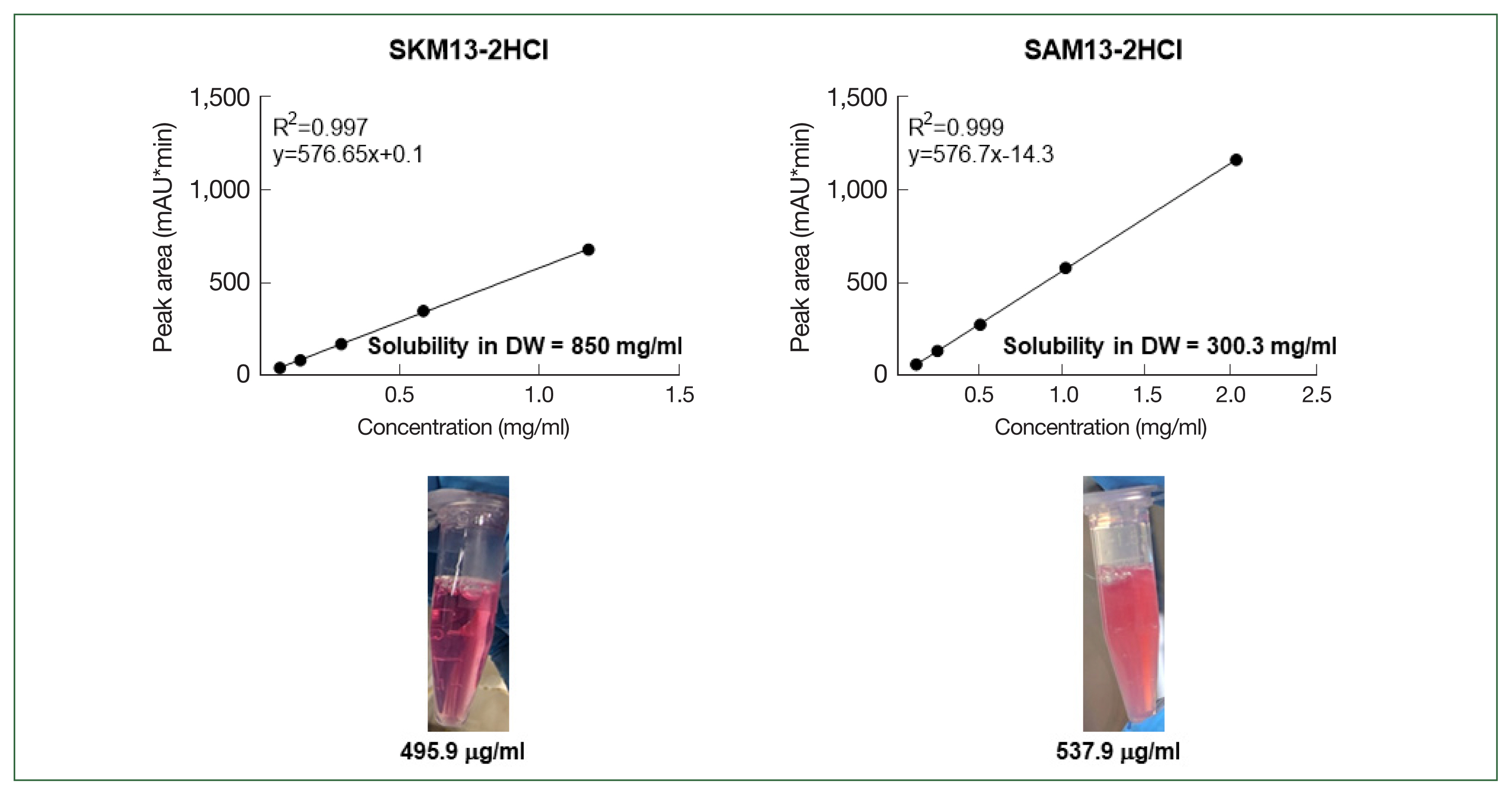
Linear regression analysis was performed to determine the solubility of SKM13-2HCl and SAM13-2HCl in deionized water. Standard samples of each substance were prepared at 5 different concentrations, and the UV absorbance was measured at 342 nm during HPLC (Supplementary Fig. S2). To ensure that the substance was persistently present as a salt in the HPLC column, 1% trifluoroacetic acid (in water) was mixed with the HPLC solvent.
The concentrations of the saturated aqueous solutions of both samples were calculated using linear regression equations (Fig. 4). The solubility of SKM13-2HCl and SAM13-2HCl was 850.8 and 300.3 mg/ml. The lower panel of Fig. 4 shows the turbidity of 1 mM of each drug dissolved in cell culture media (DMEM); SAM13-2HCl was turbid, whereas the culture medium with SKM13-2HCl was transparent.
Discussion
Antimalarial drugs, including quinine and CQ, act on the blood forms of the malaria parasite to clear the parasite; primaquine is also used to eliminate hypnozoites in South Korea [16–18]. Therefore, we developed novel molecules with a CQ structural template, including a quinolone moiety and a modified side chain (e.g., α, β-unsaturated amide) such that SKM13 could be a used in a strategy to further develop antimalarial drugs [6].
SKM13 was identified as being effective as an antimalarial candidate; however, mice exhibited discomfort when administered an i.v. injection a high-concentration solution of SKM13 in DMSO. Preparation of SKM13 in the salt form (SKM13-2HCl) successfully increased the survival rate compared with that obtained with SKM13 template [7]. Given that 1 mode of action of SKM13-2HCl involved activating the phosphorylation of eIF2α [7], the hallmark of endoplasmic reticulum (ER) stress, SKM13 derivatives were expected to be toxic and kill parasites. ER stress has been implicated as a potential drug target for inhibiting parasite survival [19]. However, SKM13-2HCl was also highly hydrophilic in salt form and exhibited less adsorption after oral administration than SKM13 [7].
In the current study, we aimed to improve the balance between hydrophilicity and hydrophobicity by modifying the SKM13-2HCl structure. The newly synthesized compounds were classified into 3 main groups (see Materials and Methods). The purpose of synthesizing derivatives after removing the double bond from SKM13 was to determine the antimalarial potency of simpler derivatives. Removal of the double bond was expected to substantially effect drug activity because this reduces the molecule size and considerably alters the polarity. Therefore, SAM09-2HCl and SAM11-2HCl were expected to be less cytotoxic than SKM13-2HCl, which was confirmed in our results (Table 1); however, their cytotoxicity was noncomparable with that of CQ. Both SAM09-2HCl and SAM11-2HCl where synthesized without the SKN13 double bond, and the cytotoxicity of SAM11-2HCl with an ethyl group did not significantly differ from that of SAM09-2HCl, which contained a methyl group.
As a second strategy, 3 different SKM13 derivatives containing N, N-diethyl group or a ring, such as piperidine or morpholine (SAM10-2HCl, SAM12-2HCl, and SAM13-2HCl), were synthesized because piperidine or morpholine moieties can exert nontoxic antitumor activities [20]. Only SAM13-2HCl, which contained a morpholine moiety, had less cytotoxicity than SKM13-2HCl, although this was comparable with that of CQ, whereas the N, N-diethyl derivative (SAM10-2HCl) and piperidine (SAM12-2HCl) were more toxic than SKM13-2HCl.
In the last 4 derivatives (SAM14-HCl, SAM15-HCl, SAM16-HCl, and SAM17-HCl-TFA), the ethylene diamine was altered to a glycine unit, a structural feature that can increase polarity and limit conformational changes. Herein, only the quinoline ring can serve as a base in an acid–base reaction; therefore, unlike the other derivatives, only one form of the HCl salt is possible. The derivatives used in the biological tests were prepared in HCl, which is more soluble in water and easier to handle.
Among the 4 derivatives containing a glycine unit (SAM14-HCl, SAM15-HCl, SAM16-HCl, and SAM17-HCl-TFA), SAM14-HCl and SAM17-HCl-TFA were noncytotoxic, whereas others (SAM15-HCl and SAM16-HCl) with only alkyl units were toxic. Interestingly, 3 less toxic candidates (SAM13-2HCl containing morpholine and SAM14-2HCl and SAM17-HCl-TFA with a glycine unit) possessed CC50 values comparable with that of CQ and differed significantly in terms of suppressing 2 human-infectious malaria parasites (P. falciparum 3D7 and K1 strains).
Both P. falciparum strains (3D7 or K1) were equally susceptible to inhibition by SAM13-2HCl with efficient IC50 values. The final SI (CC50/IC50) of SAM13-2HCl was 3-fold higher than that of SKM13-2HCl for both strains, indicating that SAM13-2HCl had a more effective antimalarial activity than SKM13-2HCl. Unexpectedly, SAM13-2HCl showed lower efficacy in the parasite-infected rodent model than SKM13-2HCl, despite the in vitro SI value of SAM13-2HCl being higher than that of SKM13-2HCl. Pathological examination of the spleen and liver confirmed the limitations of SAM13-2HCl in the animal model.
Considering the solubility and PK analyses, the reduced polarity of SAM13-2HCl could decrease the effective antimalarial activity in vivo. This finding implies that the increased number of carbons in SAM13-2HCl substantially enhanced lipid solubility. Additionally, the greater hydrophobicity of SAM13-2HCl compared with that of SKM13-2HCl could explain the lower Cmax of SAM13-2HCl, and the more hydrophobic SAM13-2HCl may therefore aggregate in the plasma, resulting in a poor antimalarial activity. In addition, the poor efficacy of SAM-13-2HCl could be attributed to variations in molecular target proteins between human-infectious P. falciparum and rodent-infectious P. berghei parasites.
In conclusion, a member of the SAM derivative family, SAM13-2HCl containing morpholine, showed a 3-fold higher efficacy in vitro against human infectious P. falciparum (3D7 and K1) parasites than that of SKM13-2HCl. However, SAM13-2HCl exerted poor efficacy in vivo compared with the SKM13-2HCl template in rodents infected with the P. berghei parasite; this could be attributed to poor solubility and less efficient PK dynamics. A more advanced hydrophobic–hydrophilic characterization of SAM13-2HCl may be the next step in the development of improved antimalarial candidates.
Supplementary Information
Acknowledgments
The following reagent was obtained through BEI Resources, NIAID, NIH: Plasmodium falciparum, Strain K1, MRA-159, contributed by Dennis E. Kyle. The following reagent was obtained through BEI Resources, NIAID, NIH: Plasmodium berghei, Strain NK65, MRA-268, contributed by Victor Nussenzweig. This research was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2015R1A6A1A03032236) and (NRF-2021R1A2C2007346).
Notes
The authors declare no conflict of interest.
Conceptualization: Kim HS, Yeo SJ
Data curation: Hong H, Moon K, Trinh TTT
Formal analysis: Hong H, Moon K
Funding acquisition: Park H, Yeo SJ
Investigation: Hong H, Moon K, Trinh TTT Methodology: Trinh TTT, Eom TH
Supervision: Park H, Yeo SJ
Validation: Yeo SJ
Writing – original draft: Kim HS, Yeo SJ
Writing – review & editing: Kim HS, Yeo SJ