Molecular detection and characterization of Acanthamoeba infection in dogs and its association with keratitis in Korea
Article information
Abstract
Acanthamoeba infection is associated with keratitis in humans; however, its association with keratitis in dogs remains unclear. To investigate this possibility, we collected 171 conjunctival swab samples from dogs with eye-related diseases (65 with keratitis and 106 without keratitis) at Chungbuk National University Veterinary Teaching Hospital, Korea, from August 2021 to September 2022. Polymerase chain reaction identified 9 samples (5.3%) as Acanthamoeba positive; of these, 3 were from dogs with keratitis (4.6%) and 6 were from dogs without keratitis (5.7%). Our results indicated no significant association between Acanthamoeba infection and keratitis, season, sex, or age. All Acanthamoeba organisms found in this study had the genotype T4, according to 18S ribosomal RNA analysis. Acanthamoeba infection in dogs might have only a limited association with keratitis.
Acanthamoeba is a free-living protozoan that is ubiquitous in environments such as dust, soil, air, tap water, and beach sand, which facilitates its access to human and animal hosts. The life cycle of Acanthamoeba has two distinct stages: vegetative trophozoite and dormant cystic. The trophozoite stage is found mainly in favorable environments in an infective form; in the cystic stage, the protozoan is resistant to harsh environmental conditions, such as extremes of temperature, humidity, and pH and chemical toxicity, which enables its global distribution [1].
The genotypes of Acanthamoeba have traditionally been divided into three categories (I–III) according to cyst size and morphological characteristics, and at least 30 species of Acanthamoeba have been identified [1]. In a recent molecular study of the 18S ribosomal RNA (rRNA) of Acanthamoeba, 23 genotypes (T1–T23) were identified, of which genotype T4 was the most common.
Not all Acanthamoeba species are pathogenic; infection with pathogenic Acanthamoeba commonly causes Acanthamoeba keratitis (AK) but rarely causes granulomatous amebic encephalitis, pneumonitis, or cutaneous infections [2]. To date, 16 Acanthamoeba genotypes are known to be associated with human diseases; of these, T4 causes the most severe clinical cases of AK. The clinical signs of AK are relatively nonspecific; however, this condition may result in severe pain, inflammation, edema, and even vision loss. A recent study showed that human AK is significantly associated with wearing contact lenses, and its prevalence is increasing in industrialized countries [3].
Acanthamoeba infections in dogs with clinical symptoms such as prostatitis, ascites/peritonitis, multisystemic infection, and granulomatous amebic encephalitis have been reported. According to results of previous studies, Acanthamoeba infections have pathogenic potential not only in humans but also in dogs [4–7]. The distribution of Acanthamoeba infection in dogs and clinical cases in humans has been investigated in few studies; however, its association with keratitis has not been analyzed [8].
In Korea, Acanthamoeba infections in humans have been associated with keratitis, meningoencephalitis, pneumonia, and meningitis [9]. In addition, Acanthamoeba has been detected in contact lens storage, tap water, and ocean sediments [10]. To the best of our knowledge, AK has not been investigated in dogs. We measured the prevalence of Acanthamoeba infection in dogs and its association with keratitis.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Chungbuk, Korea (Approval Number CBNUA-1588-21-01).
From August 2021 to September 2022, we collected conjunctival swab samples from 171 dogs with eye-related diseases at the Chungbuk National University Veterinary Teaching Hospital, Cheongju, Korea. Of these dogs, 65 had keratitis. The dogs were divided into three age groups: 1–10, 11–15, and ≥16 years. The dogs were examined in different seasons: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February; Table 1). All samples were stored at 4°C after collection and processed within 1 day.
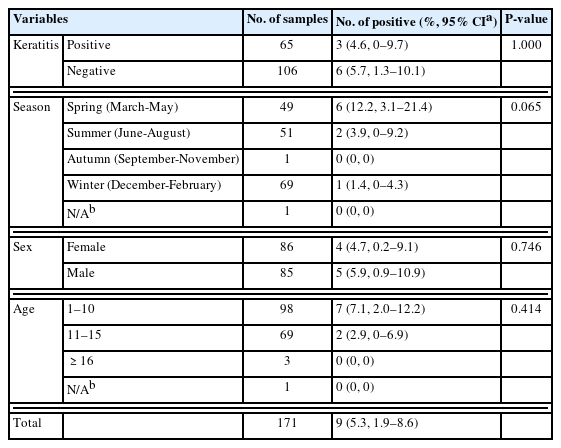
Prevalence of Acanthamoeba spp. in dogs collected at Chungbuk National University Veterinary Teaching Hospital, Korea regarding keratitis, season, sex, and age
We identified Acanthamoeba through isolation and molecular detection. Cotton swabs were mixed with 300–600 μl of 1X phosphate-buffered saline (PBS), and 200 μl of PBS was used for DNA extraction. For each dog, one cotton swab was placed on a non-nutrient agar plate containing heat-killed Escherichia coli. After culturing for 1–2 weeks, Acanthamoeba-suspected samples were placed in a peptone–yeast–glucose medium and cultured for an additional 1–2 weeks. Penicillin (50 U/ml) and streptomycin (50 μg/ml) were added to the non-nutrient agar and those in the peptone–yeast–glucose medium, and all samples were incubated at 25°C. All samples were examined daily under a microscope to confirm the presence of Acanthamoeba.
For molecular detection, we extracted genomic DNA from the mixed PBS by using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. We used a spectrophotometer (Denovix, Wilmington, DE, USA) to measure the concentration of extracted DNA. To identify Acanthamoeba, we amplified 18S rRNA by using PCR and the AccuPower HotStart PCR Premix Kit (Bioneer, Daejeon, Korea) with the JDP1 and JDP2 primer set, as previously described [11]. All Acanthamoeba-positive PCR products were sent to Macrogen (Daejeon, Korea) for direct sequencing. We used MEGA 11 software to align the obtained sequences, and we used BLAST software to compare them with sequences deposited in the GenBank database. Using the maximum likelihood method in MEGA 11, we constructed a phylogenetic tree (Fig. 1) to determine the genotype and molecular characteristics of Acanthamoeba organisms.
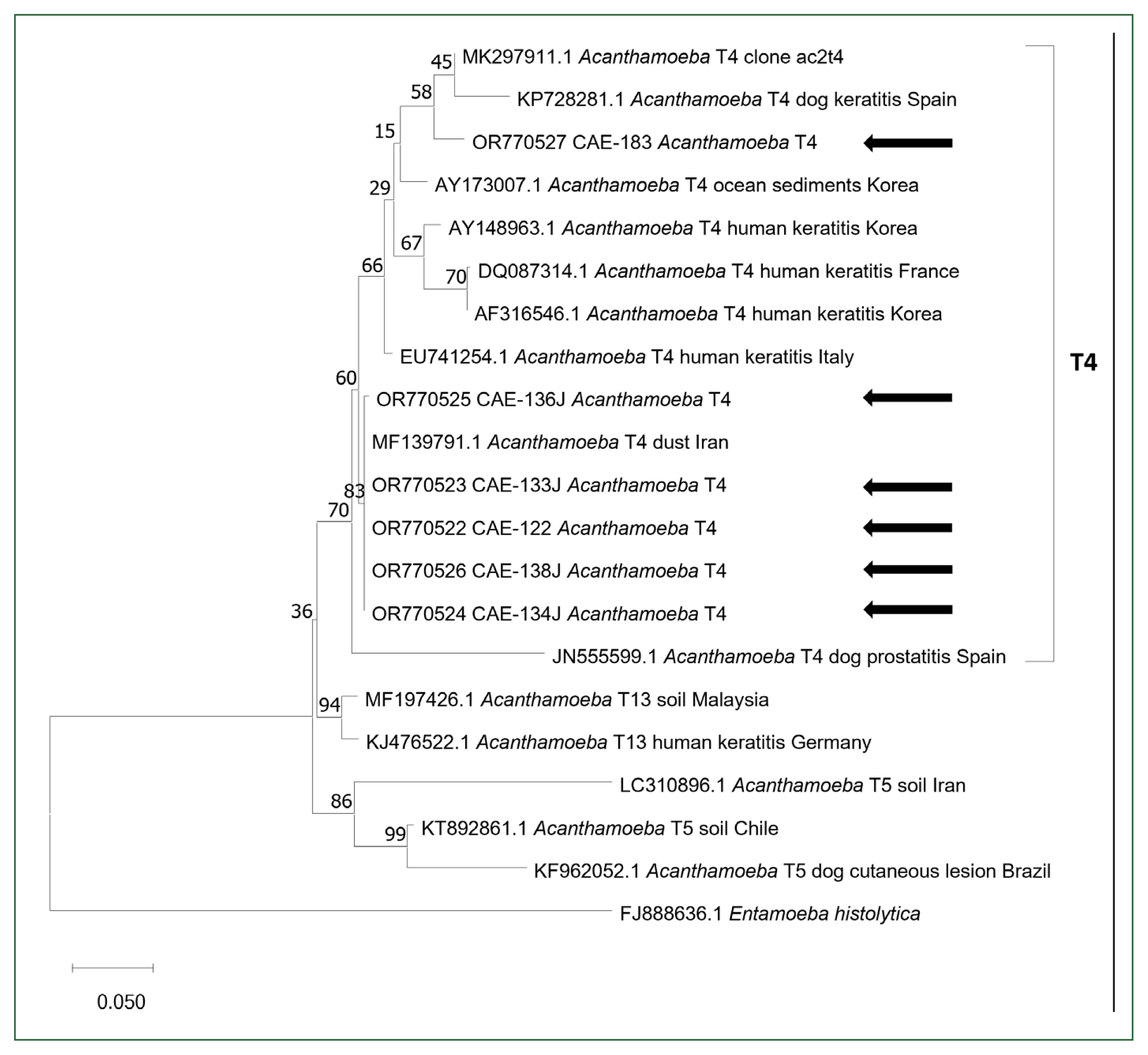
Phylogenetic tree of 18S ribosomal RNA of Acanthamoeba. To construct the phylogenetic tree, the maximum-likelihood method was used with 100 bootstrap replications. Genotypes, host, sources, and country were described. Obtained sequences in this study are indicated with black arrows.
To evaluate the association between Acanthamoeba infection and keratitis, sex, and age, we performed Fisher’s exact test with R software, version 4.3.1 (The R Foundation, Vienna, Austria). We considered P values of <0.05 statistically significant. Samples with insufficient data were excluded from the statistical analyses.
Acanthamoeba was identified by PCR in 9 (5.3%) of the 171 conjunctival swab samples (Table 1). Of these 9 Acanthamoeba-infected dogs, 3 had keratitis (4.6% of the 65 dogs with keratitis), and 6 did not (5.7% of the 106 dogs without keratitis). With regard to seasons, Acanthamoeba was identified in 6 (12.2%) of 49 samples tested during spring, 2 (3.9%) of 51 samples tested during summer, and 1 (1.4%) of 69 samples tested during winter; it was not identified in samples obtained during autumn. Four Acanthamoeba-infected dogs were female (4.7% of 86 female dogs), and 5 were male (5.9% of the 85 male dogs). Seven were 1–10 years old (7.1% of the 98 dogs in this age group), and 2 were 11–15 years old (2.9% of the 69 dogs in this age group). We observed no significant relationships between Acanthamoeba infection and the presence of keratitis, season, sex, or age (P>0.05, Fisher’s exact test).
Of the 9 Acanthamoeba-positive samples, 6 were sequenced successfully. BLAST and phylogenetic analyses revealed that all 6 sequences belonged to the Acanthamoeba genotype T4 (Fig. 1). Five showed 100% similarity to the Acanthamoeba genotype T4 (MF139791), isolated from dust in Iran, and 1 showed 97.98% similarity to the Acanthamoeba isolate T4 clone ac2t4 (MK297911). All sequences obtained in this study have been deposited in the GenBank database (OR770522-OR770527).
Acanthamoeba was isolated both on non-nutrient agar plates and in the peptone–yeast–glucose medium, while no Acanthamoeba organisms grew in culture.
To date, 23 Acanthamoeba genotypes have been identified on the basis of 18S rRNA sequence [12–18]. Identifying the genotypes of Acanthamoeba is crucial because each genotype produces different clinical symptoms and necessitates different treatment approaches [3]. Among the genotypes, T4 was the most common in both the environmental and clinical specimens in other studies. All sequences obtained in this study were identified as T4.
According to previous studies, Acanthamoeba infections have been found in dogs with different clinical signs, including prostatitis, ascites, multisystemic infections, and granulomatous amebic encephalitis. However, these clinical signs do not always correspond to those observed in humans. In addition, few clinical cases of AK in dogs have been reported; therefore, establishing a definite correlation between Acanthamoeba infections and keratitis in dogs has been difficult [8].
In this study, among the 65 dogs with keratitis, only 3 had Acanthamoeba infections; this rate was not statistically significant. Keratoconjunctivitis sicca (KCS) was the most common underlying cause of keratitis, found in 19 of the 65 dogs with keratitis in this study, followed by corneal ulcers, found in 10 dogs (data not shown ). Other causes of keratitis included lens-induced uveitis, corneal edema, and glaucoma-related inflammation. All cases of Acanthamoeba infection in the dogs with keratitis were associated with KCS. Although Acanthamoeba was also detected in dogs without keratitis, KCS may be a potential risk factor for Acanthamoeba infection in dogs’ corneas; however, no such association in dogs has been reported. A previous study showed that KCS is a risk factor for Acanthamoeba infection in humans [19]. This is probably related to the reduction in tear volume, which impairs corneal cleansing and thereby leads to microorganism overgrowth; thus, Acanthamoeba infection may be associated with eye hygiene. In our study, the prevalence of Acanthamoeba infection among all 171 dogs (5.3%) was lower than that reported for stray dogs in Spain (14.7%; 27 of 184) [20]. In that study, most Acanthamoeba-positive dogs did not show any clinical signs. The prevalence in our study might have been lower because the dogs were companion pets. On the basis of these results, we suggest that Acanthamoeba is ubiquitous and can infect dogs; however, infection is not significantly correlated with clinical indications.
This study had several limitations. Although the dogs were categorized as having or not having keratitis, keratitis was not definitively diagnosed. Because corneal trauma is a well-known risk factor in human AK [3], it carries similar risk in dogs. Therefore, a definitive diagnosis should be made to identify whether the cause of keratitis is traumatic or infectious, such as bacteria or fungi.
To the best of our knowledge, this is the first report of Acanthamoeba infection in dogs in Korea, which is associated with keratitis. However, because of the limited number of Acanthamoeba-positive cases, further well-designed studies with larger samples are necessary. In addition, clinicians should attempt to isolate Acanthamoeba from dogs with keratitis.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; No. 2021R1F1A1061795) and the Basic Research Lab Program (2022R1A4A1025557), funded by the Ministry of Science and ICT in Korea.
Notes
The authors declare no conflict of interest related to this study.
Conceptualization: Park KM, Lee SH
Data curation: Lee S, Alkathiri B, Jung JS, Kang N, Hwang J, Park SE, Hong YC
Formal analysis: Lee S, Alkathiri B, Hong YC
Funding acquisition: Park KM, Lee SH
Investigation: Alkathiri B, Jung JS, Kang N, Hwang J, Park SE
Project administration: Lee SH
Software: Lee S
Supervision: Lee SH
Validation: Hong YC
Writing – original draft: Lee S
Writing – review & editing: Park KM, Lee SH
