Organ-specific Toxocara canis larvae migration and host immune response in experimentally infected mice
Article information
Abstract
We investigated organ specific Toxocara canis larval migration in mice infected with T. canis larvae. We observed the worm burden and systemic immune responses. Three groups of BALB/c mice (n=5 each) were orally administered 1,000 T. canis 2nd stage larvae to induce larva migrans. Mice were sacrificed at 1, 3, and 5 weeks post-infection. Liver, lung, brain, and eye tissues were collected. Tissue from 2 mice per group was digested for larval count, while the remaining 3 mice underwent histological analysis. Blood hematology and serology were evaluated and compared to that in a control uninfected group (n=5) to assess the immune response. Cytokine levels in bronchoalveolar lavage (BAL) fluid were also analyzed. We found that, 1 week post-infection, the mean parasite load in the liver (72±7.1), brain (31±4.2), lungs (20±5.7), and eyes (2±0) peaked and stayed constant until the 3 weeks. By 5-week post-infection, the worm burden in the liver and lungs significantly decreased to 10±4.2 and 9±5.7, respectively, while they remained relatively stable in the brain and eyes (18±4.2 and 1±0, respectively). Interestingly, ocular larvae resided in all retinal layers, without notable inflammation in outer retina. Mice infected with T. canis exhibited elevated levels of neutrophils, monocytes, eosinophils, and immunoglobulin E. At 5 weeks post-infection, interleukin (IL)-5 and IL-13 levels were elevated in BAL fluid. Whereas IL-4, IL-10, IL-17, and interferon-γ levels in BAL fluid were similar to that in controls. Our findings demonstrate that a small portion of T. canis larvae migrate to the eyes and brain within the first week of infection. Minimal tissue inflammation was observed, probably due to increase of anti-inflammatory cytokines. This study contributes to our understanding of the histological and immunological responses to T. canis infection in mice, which may have implications to further understand human toxocariasis.
The larvae of Toxocara canis and T. cati cause human toxocariasis (visceral larva migrans) which is one of the most widespread zoonotic infections globally [1,2]. The T. canis larvae was found in liver biopsies, which was the first identification of this parasite in human disease [3]. Subsequent studies solidified T. canis and T. cati as culprits of toxocariasis. Humans and other animals, including chickens, pigs, and rodents, serve as paratenic hosts for Toxocara [2]. In these hosts, the larvae cannot develop into adults. Thus, animal models are invaluable for studying the pathological mechanisms of toxocariasis.
Toxocariasis primarily occurs through 2 routes: i) ingestion of Toxocara eggs in contaminated soil; or ii) ingestion of raw/undercooked meat of paratenic hosts harboring larvae [2,4]. Following ingestion, larvae penetrate the intestinal walls, enter the bloodstream, and migrate to the liver. From the liver, they further migrate to various organs, particularly to the central nervous system (CNS), causing diverse larva migrans symptoms and triggering host inflammatory responses [1,5]. Ocular toxocariasis, a unique manifestation, arises when larvae migrate to the posterior compartment of the eye, sparing the anterior segment [6].
Experimental T. canis infections reveal a complex interplay of host immune responses involving innate inflammation and the Th17/Th2 pathways; however, interleukin (IL)-10, tumor necrosis factor-alpha (TNF-α), or interferon (IFN)-γ are not significantly increased after infection. Increase in peripheral leukocytes, especially neutrophilia followed by eosinophilia, is also a notable feature of toxocariasis [7].
Since the first histological study of T. canis, significant progress has been made in understanding larval migration, including behavioral and pathological changes caused by the larvae [2,5,8,9]. However, experimental reports on the ocular T. canis leukocytes larval burden are lacking, compared to those on other organs. Additionally, studies on the ocular tissue-specificity of the migrating larva and the histology of ocular toxocariasis remain few. Here, we address these knowledge gaps by examining T. canis larval migration and load in various organs, including the eyes. We also examine the histological features of the infected tissues and the host systemic immune response during the first 5 weeks of T. canis infection.
BALB/c mice were chosen for this study due to their known host-parasite interaction characteristics listed here: enhanced parasite larval migratory capacity, high susceptibility to parasite infection, and higher accumulation of parasite larvae in the brain [9]. Three groups of 6-week-old male BALB/c mice were orally infected with 1,000 T. canis 2nd stage larvae each. T. canis larvae used for infection were obtained as follows: Adult T. canis worms were retrieved from the feces of naturally infected dogs. Eggs were extracted from the uteri of adult female worms through mechanical maceration and subsequently cultured in flasks containing 50 ml of 1% neutral formalin for 21 days at 27°C with regular inspection. The embryonated eggs were then preserved in 0.1 M H2SO4 at 4°C until further use. Five mice in each group received the single-dose inoculation. Mice in groups 1, 2, and 3 were sacrificed 1, 3, and 5 weeks post-infection, respectively. An additional 5 non-infected mice served as controls. In this study, both experimental and control animals were co-housed under specific pathogen-free conditions. All mice were provided ad libitum water and food and maintained in a temperature-controlled environment (22–23°C) with a 12-h light/12-h dark cycle.
We adhered to the Association for Research in Vision and Ophthalmology Statement (ARVO Statement) for the Use of Animals in Ophthalmic and Vision Research in our animal experiments. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (Permit number: IACUC: BA1109-090-057-01).
The liver, lungs, brain, and eye tissues from 2 mice in each group were finely minced. Larvae were recovered from each organ through digestion using artificial gastric juice (0.1% hydrochloric acid and 0.5% pepsin) (Sigma-Aldrich, St. Louis, MO, USA). Specifically, to recover the infecting parasite larvae, the tissues were incubated overnight in the artificial gastric juice at 37oC. The digests were centrifuged for 2 min at 1,500 rpm. The resulting sediments were collected, transferred to a petri dish, and examined under a stereoscopic microscope.
Tissue samples from the liver, lungs, brain, and eyes, of the remaining 3 mice in each group, were fixed in 10% formaldehyde, dehydrated with ethanol, and embedded in paraffin. Tissue sections (4-μm thickness) were stained with hematoxylin-eosin and examined under a standard light microscope.
Blood samples were collected from the tail vein of each mouse at 1, 3, and 5 weeks post-infection using EDTA-coated microvette tubes. An automated blood cell analyzer (HemaVet 950, Drew Scientific Inc., Waterbury, CT, USA) was used to obtain complete blood counts, which provided insights into potential changes in white blood cell populations due to T. canis infection.
At 3 and 5 weeks post-infection, 1 ml of bronchoalveolar lavage (BAL) fluid was collected from the lungs of each mouse. Following centrifugation at 400×g for 10 min at 4°C, the supernatants were stored for subsequent cytokine assays. The levels of IL-4, IL-5, IL-10, IL-13, IL-17, and IFN-γ in the BAL fluid were measured using commercially available sandwich ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Analyzing these specific cytokines helps elucidate the profile of the immune response triggered by the T. canis infection.
Statistical evaluation was performed using R ver. 4.3.2 (The R Foundation for Statistical Computing). Measurements were compared using the Kruskal–Wallis test with Dunn’s test as a post-hoc analysis. The Bonferroni correction was applied for multiple comparisons of the formulas. Statistical significance was set at P<0.05.
One week after infection, the average number of larvae discovered in the liver, lungs, brain, and eyes was 72±7.1, 20±5.7, 31±4.2, and 2±0, respectively (Fig. 1). These numbers were not significantly different at 3 weeks post-infection (58.5±7.8, 20.5±6.4, 29±2.8, and 2±1.4, respectively). However, at 5 weeks, the liver and lung tissues showed a decrease in larvae numbers to 10±4.2 and 9±5.7 respectively; whereas the larvae numbers in the brain and eyes stayed constant (18±4.2 and 1±0, respectively).
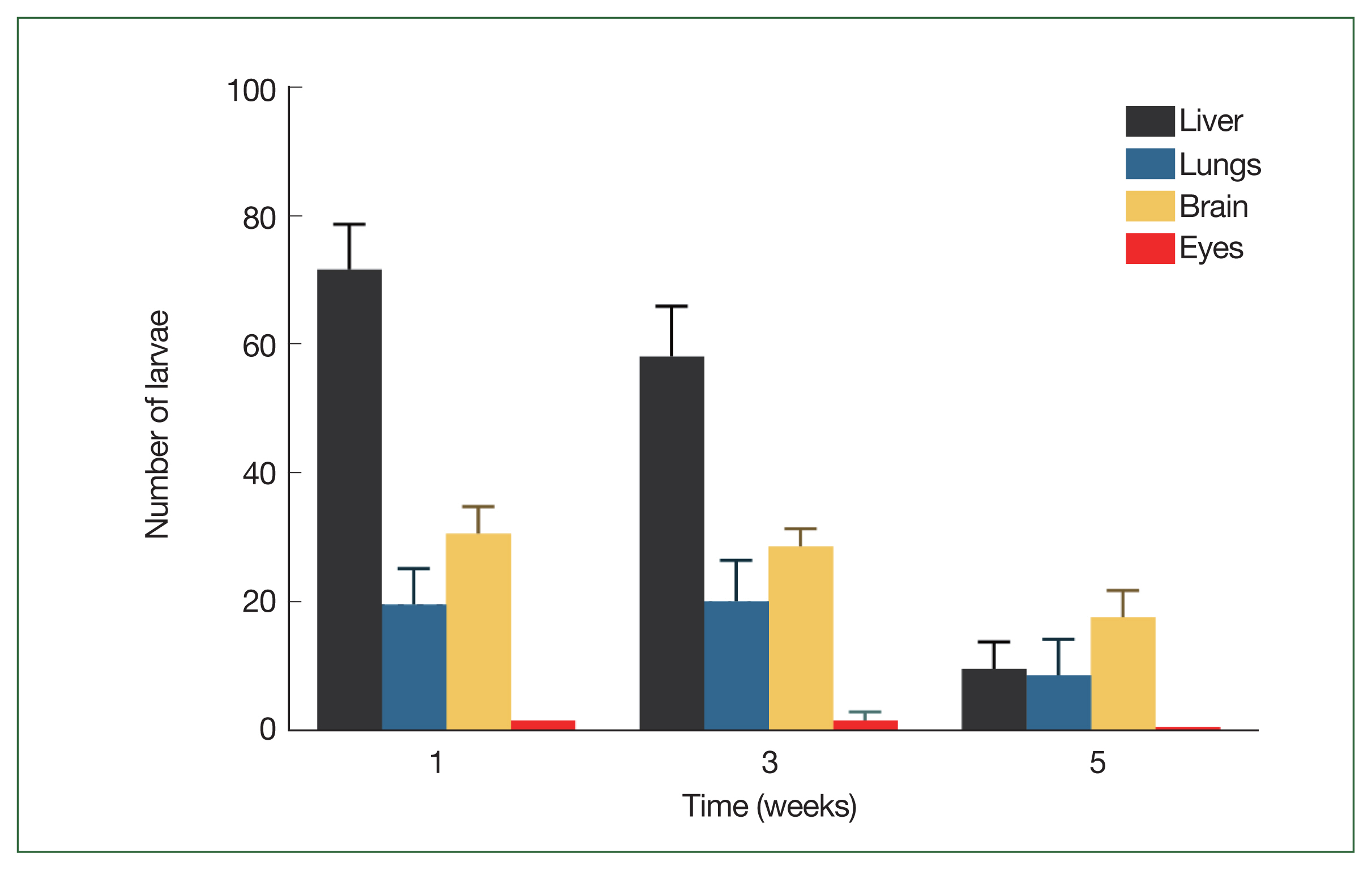
Migratory behavior of Toxocara canis larvae in BALB/c mice. Mean number of total Toxocara canis larvae recovered from 4 organs of BALB/c infected with a 1,000-larvae dose after 1-, 3-, and 5-week post-infection.
Histopathologic analysis of the retina at 1 week post-infection showed a tubular structure consistent with Toxocara larvae in both the middle and outer layers, with minimal surrounding inflammation (Fig. 2A–C). However, when T. canis larvae were present in the inner retina, eosinophilic infiltration was observed surrounding the vitreous and the inner retinal surface (Fig. 2D, E). Eosinophilic inflammation was also minimal in the liver tissue (Fig. 2F), but prominent in the lung tissues (Fig. 2G). Finally, T. canis larval invasion was confirmed in the brain, but without accompanying inflammation (Fig. 2H, I).

Histopathological findings of the Toxocara canis larvae infected tissue. Toxocara canis larvae (arrows) in the retina (A–E), liver (F), lung (G), and brain (H and I) in the first week post-infection. Note that Toxocara canis larvae are present in all layers of retina. Compared to the larvae in the outer or middle retina (A–C), the larvae in the inner retina (D and E) recruit multiple eosinophils in the vitreous and the inner surface of the retina. Eosinophil infiltration (arrowhead) was markedly noted in the lung (G) (H & E, 200× magnification; Scale bar: 100 μm). Magnified images of larvae in the retina (C and E) and brain (I) (400× magnification; Scale bar: 20 μm).
Compared to that in the control group, the blood of the infected mice group showed elevated neutrophils and monocytes in the first and third weeks, while eosinophils were elevated in the first week (all P<0.017, Fig. 3A, B). However, these cells gradually declined in number throughout the 5 weeks post-infection observation period.
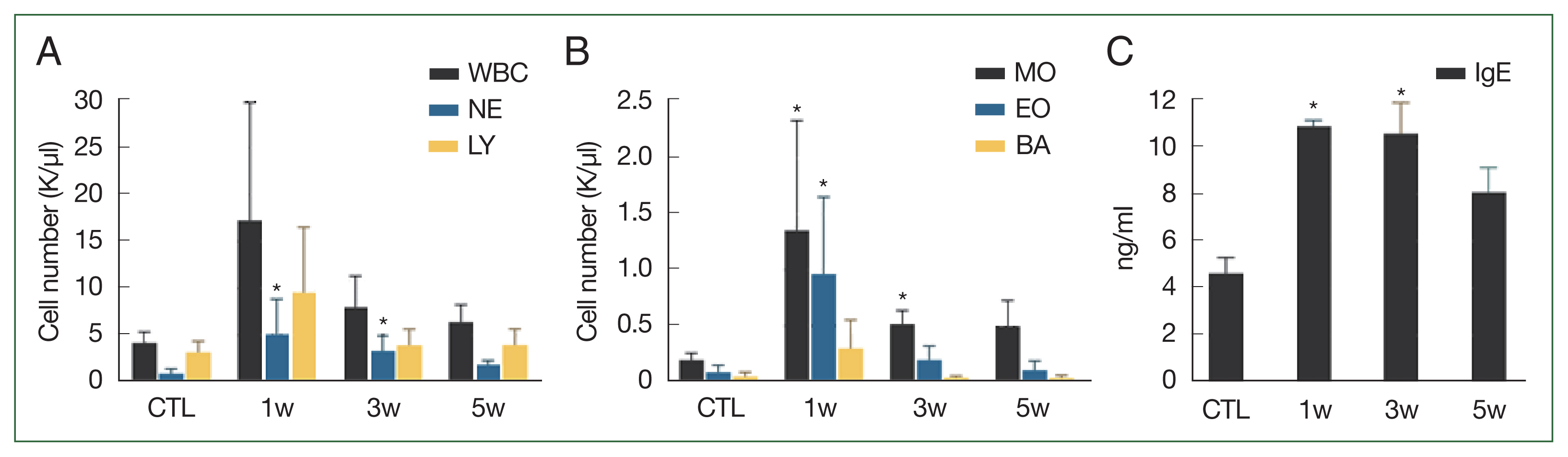
Changes in blood cell count and antibodies in blood in response to Toxocara canis larval infection. Differential blood cell counts in control and infected mice (A, B); Serum IgE titers were higher in infected mice than control mice at 1, 3, and 5 weeks (C). Significant differences compared to control group are indicated by an asterisk (P<0.017, Kruskal–Wallis test). CTL, control mice; INF, infected mice; NE, neutrophil; LY, lymphocyte; MO, monocyte; EO, eosinophil; BA, basophil.
Serum immunoglobulin E (IgE) titers evaluated in the first- and third-week post-infection, followed a similar trend, with significantly higher levels in infected mice than controls (P=0.016, <0.001, respectively). However, the IgE titers gradually decreased over the 5 weeks of post-infection observation period (Fig. 3C).
Cytokine analysis of the BAL fluid revealed a distinct pattern in the infected group compared to control group in the third and fifth weeks. In the infected mice group, levels of IL-5 and IL-13 were significantly higher in the fifth week (P=0.005 and 0.008, respectively), whereas the levels of IL-4, IL-10, IL-17, and IFN-γ were similar to those of controls (Fig. 4).
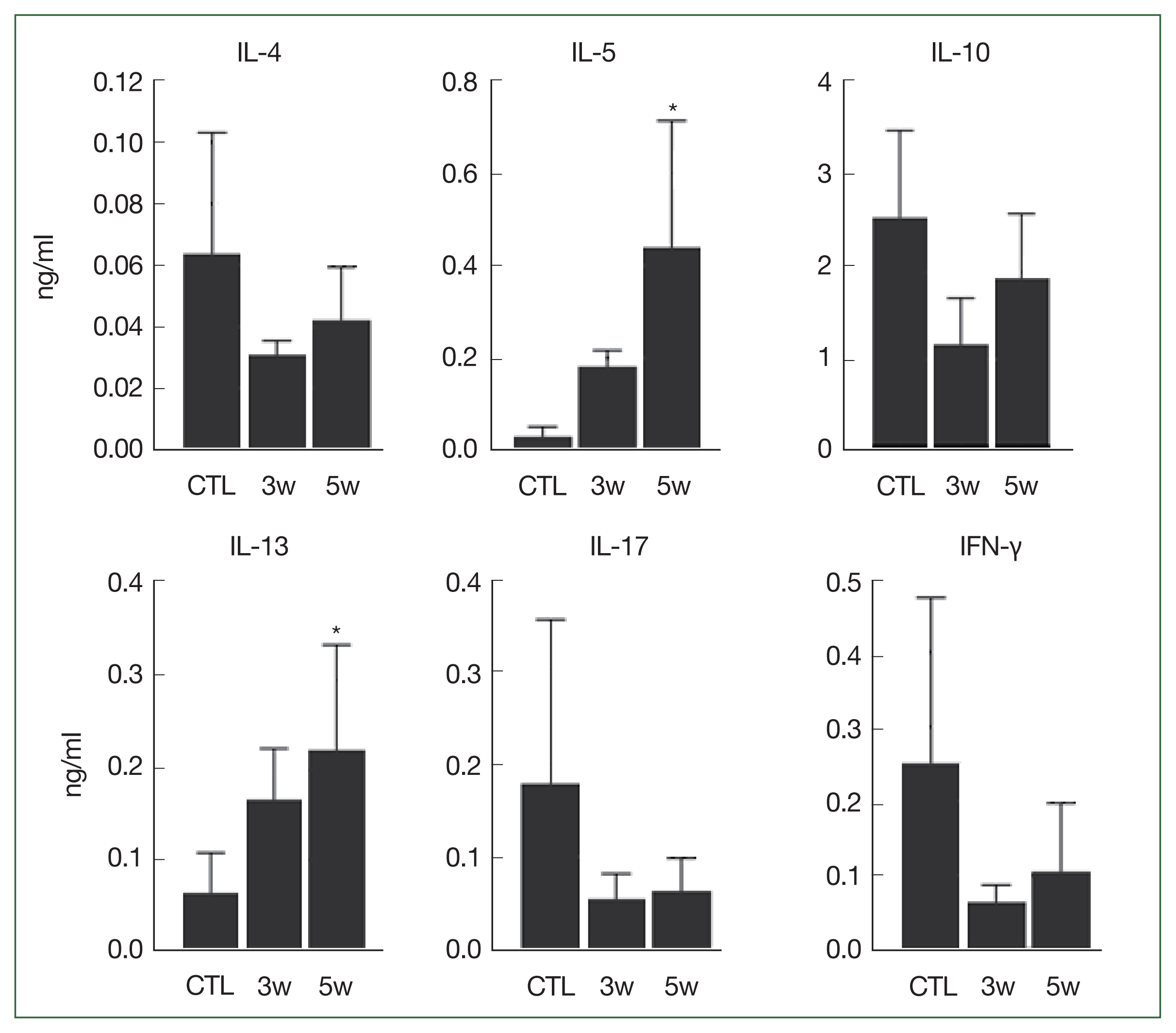
Cytokines in bronchoalveolar lavage fluid (BAL). Significant differences compared to control group are indicated by an asterisk (P<0.025, Kruskal–Wallis test). CTL, control; INF, infected mice.
Our study followed the dynamics of T. canis larval migration and systemic immune responses in mice infected with T. canis for 5 weeks. We found that, 1 week after infection, live Toxocara larvae were found in the brain (31±4.2) and eyes (2±0) and their numbers remained stable for the study duration; meanwhile the inflammation observed was minimal. While nearly all mice organs are invaded to varying degrees due to systemic circulation of the larvae, the eye is a notable exception, possibly due to its proximity to the CNS and the resemblance of retinal neural tissue to the brain. The anterior segment of the eye is typically spared, while the posterior segment is preferentially targeted, resulting in ocular toxocariasis [10,11].
Previous studies have confirmed CNS involvement in BALB/c mice infected by T. canis larvae, beginning on the third day after infection [2]. Another report, using a single 1,000–embryonated egg dose in BALB/c mice, found 15 larvae in the brain 5 days after inoculation; 7 days after inoculation 29 larvae were found in the brain and one larva was found in the eyes, which aligns with our findings [9]. Notably, their work suggests that larvae invade the eyes only after infecting the brain, implying live migration from established brain colonies. Interestingly, unlike in the gut, liver, and lungs, the number of larvae in the brain and eyes remained relatively constant, in both our study and previous reports [2,9]. This might be attributed to the varying Toxocara larvae accumulation capacities of different organs.
Only 2 previous studies have presented histopathological images of Toxocara larvae transversing retinal tissue [8,12]. Here, we present further high-quality histological images of mouse retinal tissue harboring Toxocara larvae. Interestingly, while larvae residing in the inner retina triggered eosinophilic inflammation, those in the middle and outer layers remained quiescent. We previously established that retinal granuloma is a hallmark of ocular toxocariasis, with its presence across all retinal layers [13]. However, frequent occurrence of T. canis in epiretinal membranes suggests a predominant involvement of the inner retina. Our current histopathological findings offer the following insights into the potential pathogenesis of human retinal granuloma: Toxocara larvae migrating into the inner retina recruit eosinophils to the vitreous and inner retinal surface, leading to larval encapsulation and chronic inflammation in the surrounding area. To our knowledge, the mechanisms underlying retinal granuloma formation in ocular toxocariasis remain uncharacterized. Long-term follow-up of histopathological findings in future studies could shed light on this critical aspect of the disease process.
We also observed activation of neutrophils, monocytes and eosinophils, and high levels of IgE secretion, consistent with other helminthic infections [14]. These findings align with previous reports on Toxocara-infected mice, where inflammatory cells were detected in various organs and peripheral blood [7,15]. Resende et al. [7] reported elevated neutrophil counts in peripheral blood, peaking at 7 days of post-infection, suggesting their role in the early innate response against the parasite, with counts returning to baseline by 14 days after infection. Previous studies in Toxocara-infected mice demonstrated a biphasic increase in eosinophilia (also considered part of the innate immune response), with peaks around 7–10 days and 2–3 weeks post-infection [7]. Our findings, however, revealed a peak in eosinophil count in the first week, followed by a continuous decline in the third and fifth weeks of infection. IgE antibody showed a marked and sustained elevation in serum levels throughout the 3-week infection period (Fig. 3C). We believe that this is attributed to the change in larval burden in mice over time [9].
Building upon our histopathological findings, we investigated the pulmonary immune response during acute toxocariasis. Helminth infections (such as by T. canis) trigger a shift from a Th1 to Th2 immune response, characterized by increased levels of cytokines like IL-4, IL-5, and IL-13, alongside suppression of pro-Th1 cytokines like TNF-α, IFN-γ, and IL-17 [14]. Previous studies using peripheral blood samples from T. canis-infected mice reported elevated levels of IL-4, IL-5, IL-13, IL-33, IL-6, and IL-17, with no significant changes in IL-10, TNF-α, or IFN-γ, suggesting Th2 dominance [7]. Our analysis of BAL fluid also supports T. canis induced Th-2 polarization, with increased IL-5 and IL-13 5 weeks after infection, without significant changes in IL-4, IL-10, IL-17, and IFN-γ. The shift from Th1 to Th2 immune response and changes in secreted cytokines—seen, here, in our acute toxocariasis model—likely protect the host from severe inflammation. This process is crucial in inducing host tolerance, facilitating helminth survival [14]. This immunological phenomenon is called “immune privilege,” which appears to partially explain the lack of larval infection associated inflammation in brain tissue. Othman et al. [16] reported a lack of inflammatory reactions around migrating T. canis larvae in mouse brain tissue, with no signs of vascular congestion. They proposed that increased levels of anti-inflammatory cytokines, particularly IL-6, may confer neuroprotective and neurotrophic effects.
A previous study found increased levels of IL-10 in the lungs on post-infection days 7 and 14 [17], whereas another study observed elevation of IL-10 in the peripheral blood 14 days post-infection [7]. Jiang et al. [18] have reported a significant increase in the expression of aqueous humor IL-10 in human ocular toxocariasis. Here, IL-10 elevation was not observed in BAL fluid even 5 weeks after infection, indicating that the expression of regulatory cytokines may vary depending on the infection site [7], and the duration of expression may differ for each cytokine. In a study by Pinelli et al., the IL-4 levels in BAL fluid and spleen cells showed no significant difference at any time-point after T. canis infection compared to non-infected controls [19]. However, another study reported increased production of IL-4 in lung and spleen cells derived from T. canis-infected mice [20]. In our study, serum IL-4 levels remained consistently low and unchanged, before and after infection. Further studies are required to elucidate the role of IL-4 in Toxocara infection.
The discrepancy in observed immune responses between the studies may be attributed to several factors, including (i) differences in cytokine measurement methods (ELISA or polymerase chain reaction), (ii) variations in sampling time-points, and (iii) variations in organ-specific immune responses.
Our study has several limitations. First, the experimental animal sample size was restricted owing to ethical concerns, thereby limiting the generalizability of the results from the statistical analysis. However, despite limited quantitative results, in this brief communication focusing on the T. canis larval migration in mice, we have obtained good quality informative images of tissues infected with T. canis larvae. Second, we assessed the inflammatory response especially focused on lungs (by measuring cytokines in BAL fluid) without comparing it with the systemic inflammatory response. Future investigations should aim to elucidate organ-specific inflammatory responses and conduct comparative analyses of systemic inflammatory responses in a larger number of mice.
In summary, our study demonstrates that, during the first week of infection with T. canis larvae, a small number of larvae migrate to the eyes and brain. Minimal tissue inflammation is seen, which may be attributed to the increase in anti-inflammatory cytokines during the infection process. Increased levels of anti-inflammatory cytokines also potentially explains the milder symptoms and signs of toxocariasis; acute toxocariasis is mostly asymptomatic. However, future long-term observational studies in larger animal cohorts are necessary to fully understand the inflammatory response in chronic toxocariasis, particularly its resemblance to human ocular toxocariasis.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00248480). The funding organization had no role in the design or conduct of this study.
Notes
The authors have no potential conflict of interest to disclosure.
Conceptualization: Jin Y, Woo SJ
Data curation: Jin Y
Formal analysis: Kim MS, Jin Y
Funding acquisition: Woo SJ
Investigation: Jin Y
Methodology: Jin Y, Woo SJ
Validation: Jin Y, Kim MS
Writing – original draft: Kim MS
Writing – review & editing: Kim MS, Jin Y, Woo SJ
