Abstract
This study was conducted to examine the pathogenesis of gill degeneration in the Korean rockfish Sebastes schlegelii, infested with the monogenean ectoparasite, Microcotyle sebastis. We collected 30 Korean rockfish from a fish farm in Tongyeong-si, Gyeongsangnam-do, and examined them with light microscopy, scanning electron microscopy (SEM), and histopathology, in March 2018. The monogenean trematode, M. sebastis, was detected in 27 Korean rockfish (90%), with the intensity of infection being 31.7 per fish. The characteristic surface ultrastructures such as tegument with transverse striations, genital atrium, genital pore, and opisthaptor with numerous clamps were observed. The worms were firmly attached to the gill lamellae using clamps from the opisthaptor, causing gill damage and degeneration. The distal part of the lamellae was ruptured by the sclerites of the clamps. The histopathological examination revealed epithelial hypertrophy, hyperplasia, and occasionally fusion of the lamellae. These ultrastructural and histopathological findings provide some understanding of the pathogenesis of gill degeneration in the Korean rockfish infested with M. sebastis.
-
Key words: Microcotyle sebastis, Korean rockfish, microstructure, histopathology, scanning electron microscope
The monogenetic trematodes (Class Monogenea) are groups of flatworms that cause ectoparasitic infestation (Phylum Platyhelminthes). They primarily reside on the skin, gills, and fins and are less frequently found in internal organs such as the gastrointestinal and urinary tracts of fish. Members of this helminth group are rarely found on aquatic invertebrates, amphibians, reptiles, and mammals [
1,
2]. These worms are hermaphrodites and do not require an intermediate host [
2]. Under natural environmental conditions, the monogenean helminths are uncontrollable, and their prevalence varies according to fish species, seasons, sea areas, and sea water temperature [
2–
4]. Under aquaculture conditions, they cause pathological effects and economic losses [
5–
7].
The Korean rockfish,
Sebastes schlegelii, inhabit the coastal areas of Korea, China, and Japan. It is one of the most commercially important fish species in Korea, and its mariculture has also increased rapidly [
8]. However, parasitic diseases in cultured Korean rockfish constitute one of the most serious problems [
9], where
Microcotyle sebastis (gill-infested monogenean) causes a major parasitic disease of net-cage-farmed Korean rockfish [
5,
6]. High cumulative mortalities of juvenile rockfish during summer due to heavy infestation with
M. sebastis are frequently observed in fish farms [
5,
6]. Mortality caused by polyopisthocotyleans is due to anemia and dyspnea [
10]. Nonetheless, there is limited information regarding the associated pathogenesis of gill degeneration in
M. sebastis-infested fish. This study was conducted to examine the pathogenesis of gill degeneration in the Korean rockfish infested with
M. sebastis employing light microscopy, scanning electron microscopy (SEM), and histopathological observations.
We collected 30 Korean rockfish (body length: 18.2±1.7 cm; body weight: 110.9±20.7 g) from a fish farm in the coastal area of Tongyeong-si in Gyeongsangnam-do, Korea, in March 2018. M. sebastis infestation was confirmed in the gills of 27 fish by light microscopy. The gills of the infested fish were fixed with 10% neutral buffered formalin for light microscopy and with 2.5% glutaraldehyde for SEM. The fixed tissues were embedded to paraffin block, after which Hematoxylin and eosin staining was performed. For SEM, the specimens containing parasites were postfixed in 1% osmium tetroxide for 2 h at 4°C. Then, the specimens were washed with 0.1 M phosphate buffer and dehydrated in a graded ethanol series. The specimens were substituted with amyl acetate 3 times, critical-point-dried in carbon dioxide, and sputter-coated with gold, followed by examination with SEM (JSM-6490LV, JEOL, Tokyo, Japan).
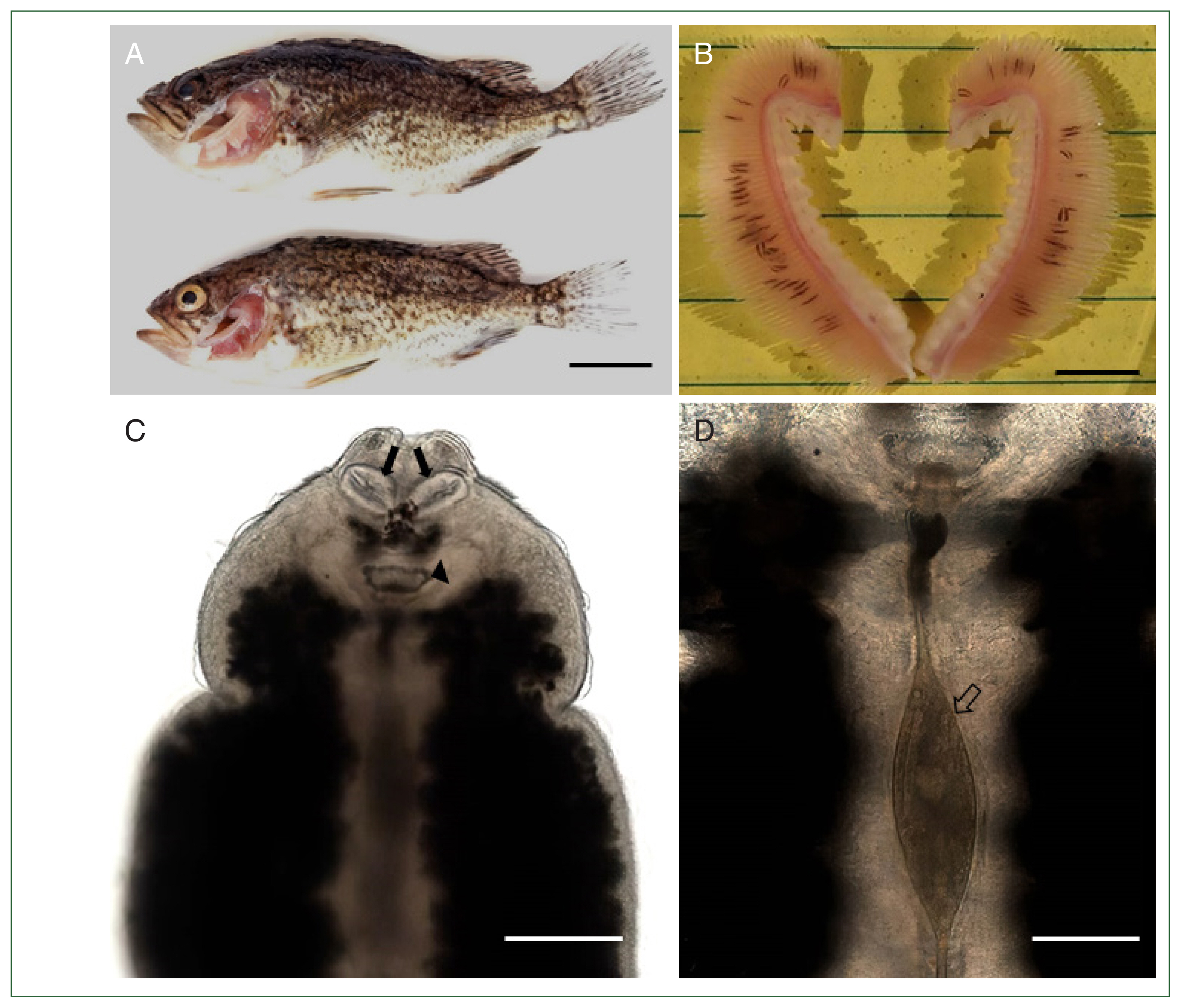
Of the 30 Korean rockfish examined, 27 (90%) were infested with
M. sebastis (mean infestation intensity of 31.7 and maximum intensity of 68). The gills of fish heavily infested with
M. sebastis showed typical clinical signs of anemia (
Fig. 1A,
B). Under the microscope,
M. sebastis showed an elongated, symmetrical body with 2 buccal suckers and a genital atrium in the anterior part (
Fig. 1C). The egg was fusiform, with a filament extending from each end (
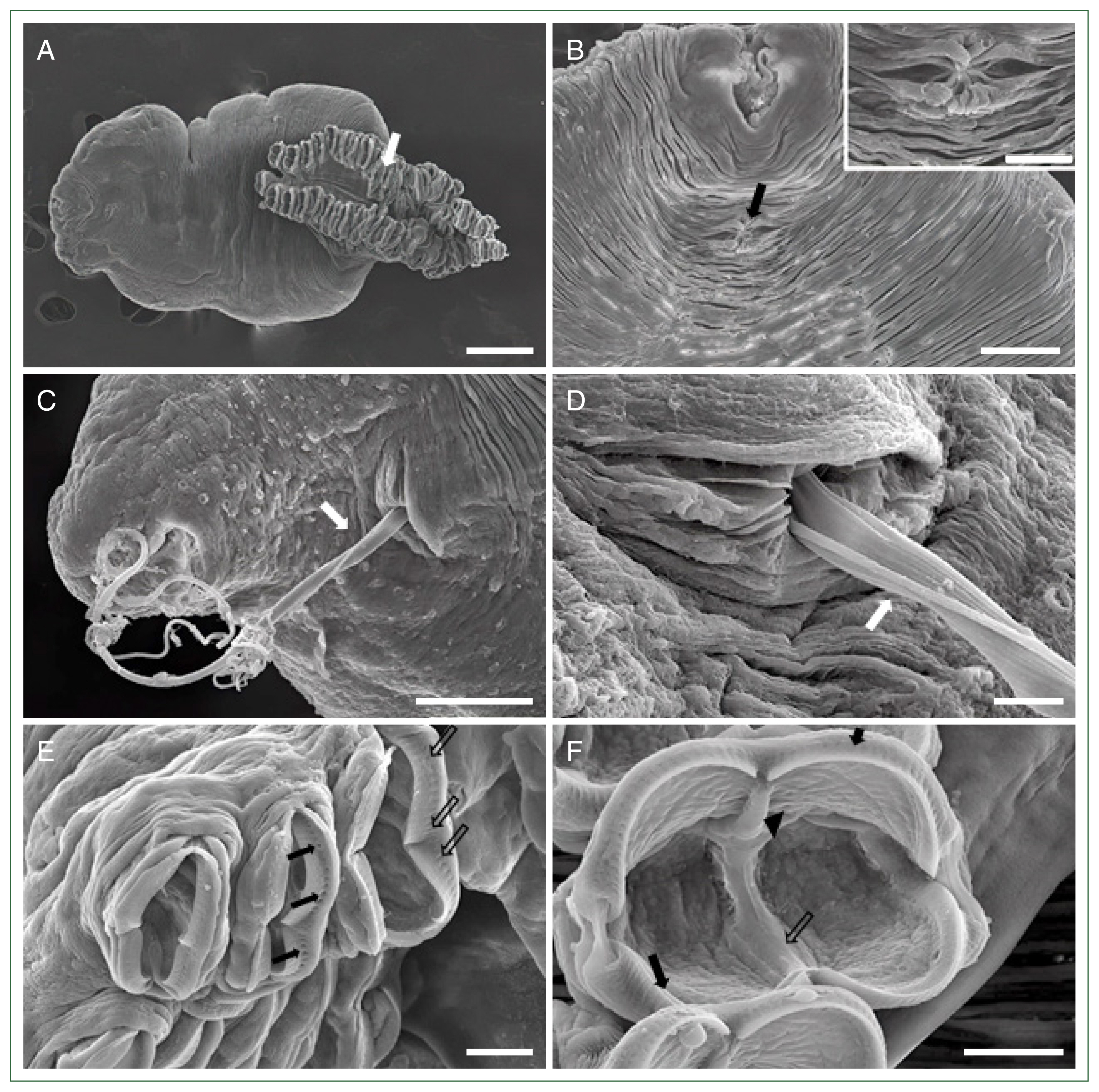
Fig. 1D). Further morphological analysis by SEM revealed the presence of a buccal cavity and a genital atrium on the ventral surface, and the body was surrounded by tegumental folds. The opisthaptor contained approximately 30 pairs of clamps arranged in 2 marginal rows attached to the gills of the host fish (
Fig. 2A). Polyp-shaped structures containing the genital atrium/pore were identified below the buccal cavity (
Fig. 2B). Moreover, the filament of the egg extruded through this genital pore (
Fig. 2C,
D). Several clamps with numerous micropits or microdepressions were observed on the surface (
Fig. 2E). Each clamp consisted of 2 pairs of lateral sclerites, curved bars forming the rim of the clamp, a ventral mid-long sclerite curved from the inside, and a dorsal mid-short sclerite connecting the median long sclerite. A muscular structure surrounded the lateral sclerites and had an irregular surface inside the clamp (
Fig. 2F). The prevalence and infestation intensity found in this study were similar to those reported previously. Although
M. sebastis infestation in cultured Korean rockfish exhibited seasonality, previous studies have reported a prevalence and intensity range during spring of approximately 93% and 0–68, respectively [
11,
12]. In previous studies, morphological characteristics of
M. sebastis were reported [
12–
15]. Among
Microcotyle species,
M. sebastis was differentially diagnosed by the position of the anterior haptoral clamp at the back of the trunk, with 23 to 31 clamps in each row [
14], which was consistent with the present study. The morphological features of
M. sebastis sclerites, including 2 pairs of lateral sclerites, long median sclerite, and short accessory sclerite, are also consistent with those reported previously [
14,
15]. Unfortunately, previous studies on the morphological properties of
M. sebastis have mainly been limited to the visualization of the ultrastructure [
12–
15]. For example, Song et al. [
12] investigated the general morphotype, the anterior prohaptor, and the posterior opisthaptor of
M. sebastis by SEM. However, our present study provides the detailed ultrastructure of the genital atrium/pore, extrusion of filaments, the clamp that includes micropits and microdepressions, and sclerites surrounded by a muscular structure based on SEM observations, which may contribute to the expansion of existing knowledge regarding the morphological characteristics of
M. sebastis.
The pathological changes induced by
M. sebastis is initiated at the site of attachment, where the parasite clamps cause damage to the gill lamellae. In this study,
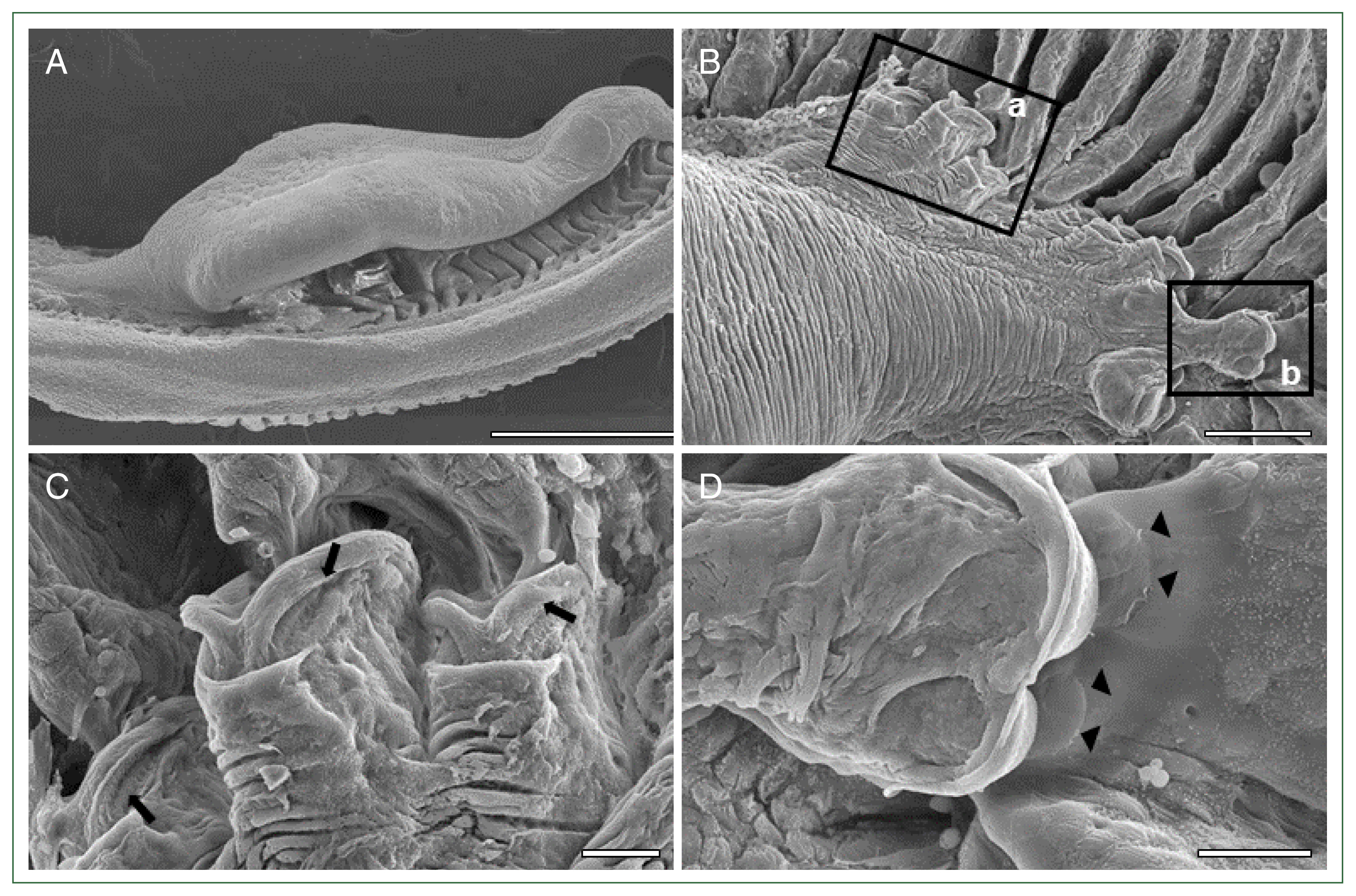
M. sebastis attached to the consecutive lamellae using numerous clamps (
Fig. 3A). These clamps grasped the distal lamella that appeared to be capable of sucking up the lamellae (
Fig. 3B, C) and caused irritation and wounds in the gill lamellae by squeezing and tweaking by the sclerites (
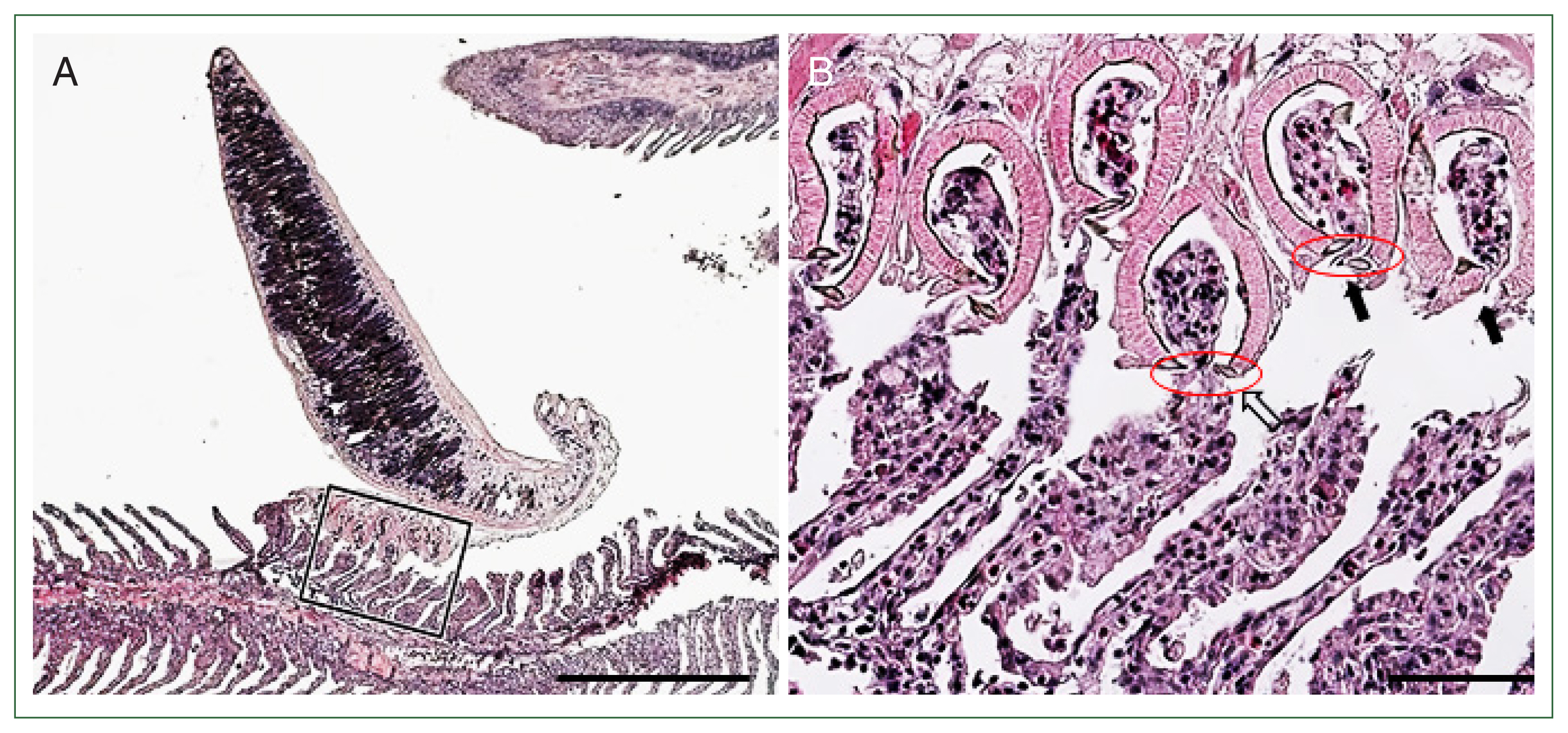
Fig. 3D). Histological examination showed that
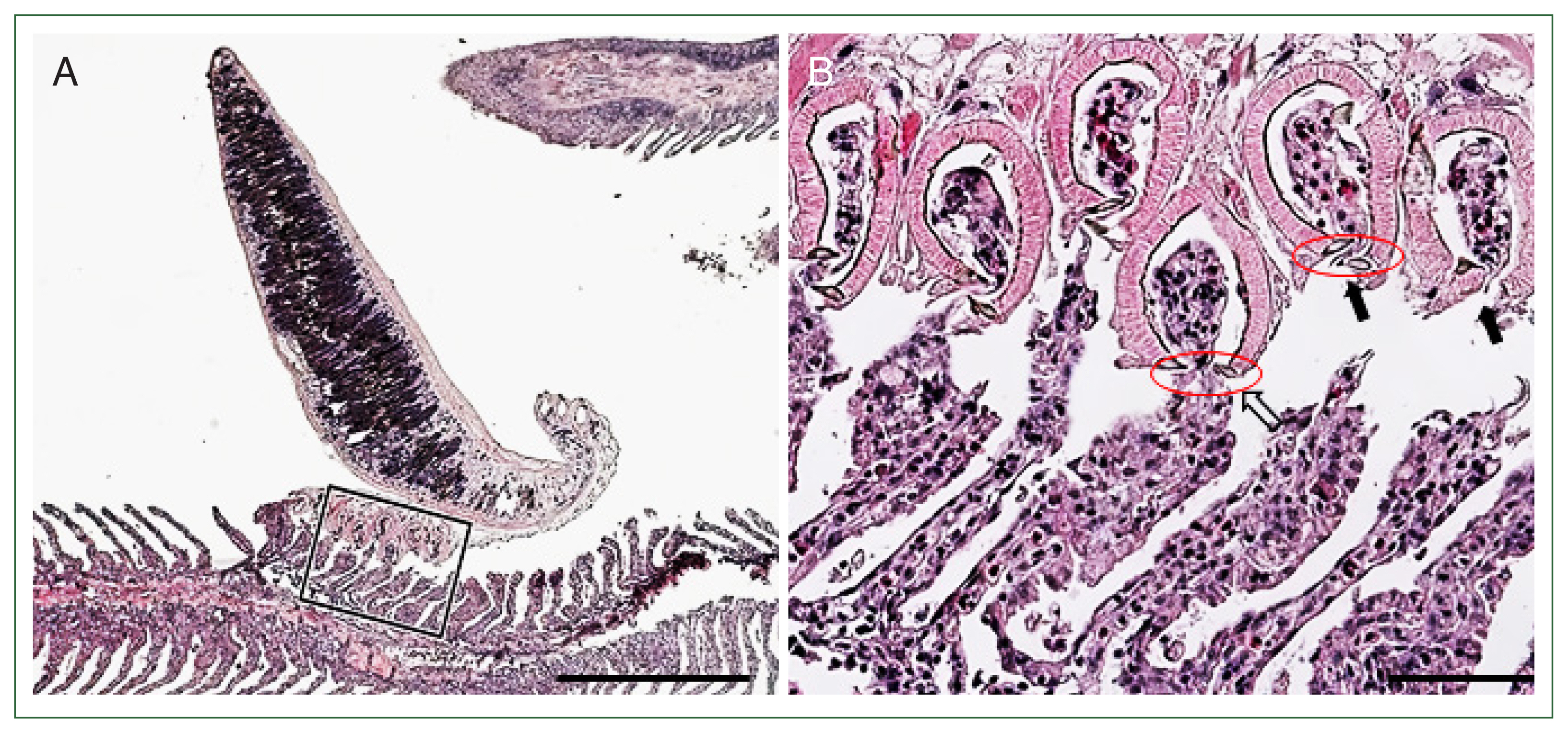
M. sebastis attached to the gill lamellae using clamps. The lamella thickness was increased compared with that at the site where the parasite was not attached (
Fig. 4A). The distal parts of the consecutive lamellae were cut or constricted by the sclerites in the lumens of the clamps. The epithelial hypertrophy, hyperplasia, and fusion of lamellae were also detected (
Fig. 4B). The mechanical damage or irritation by the parasite clamps has been reported in other monogeneans, including
Zeuxapta seriolae which infests amberjack
Seriola dumerili and
Erpocotyle tiburonis which infests the bonnethead shark
Sphyrna tiburo [
16,
17]. Although
M. sebastis is a blood-sucking parasite on fish and causes mass mortality in cultured Korean rockfish [
18], a previous study suggested that high intensities of
M. sebastis infestation weaken the host, facilitating infection by other diseases, rather than directly contributing to host mortality [
19]. Coinfection of parasites (including
M. sebastis) and pathogenic bacteria (belonging to the genera
Vibrio and
Streptococcus) have also been reported in the Korean rockfish [
9]. We speculated that the intense infestation of
M. sebastis causes severe mechanical irritation/damage to the gills, resulting in an imbalance of physiological states, secondary bacterial infection, and mortality. A limitation of this study was that the effects of gill damage on host physiological stress responses (including respiratory capacity, osmoregulation, and blood gas exchange) and the presence of pathogenic bacteria were not sufficiently investigated.
In conclusion, this study investigated the microstructure of M. sebastis and the pathological effects caused by the parasite clamps in the gills of the Korean rockfish. The detailed morphological characteristics of M. sebastis were examined. The clamps of the parasite may cause pathological changes to the gill lamellae. Further studies are required to elucidate how the gill damage affects physiological responses and secondary bacterial infection in the host.
Notes
-
Author contributions
Conceptualization: Shin SP, Kim S
Investigation: Kim S
Visualization: Shin SP, Kim S
Writing – original draft: Shin SP, Kim S
Writing – review & editing: Shin SP, Kim S
-
The authors declare no conflict of interest related to this study.
Acknowledgments
This study was supported by the research grant of the Kongju National University (2024) and also by the grant from the National Institute of Fisheries Science (R2024020).
Fig. 1(A) Two Korean rockfish
Sebastes schlegelii infested with
Microcotyle sebastis. Scale bar=3 cm. (B) Gills showing anemia caused by a heavy infestation of
M. sebastis (black lines). Scale bar=1 cm. (C) Light microscopic view of
M. sebastis showing buccal haptors (arrows) and genital atrium (arrow head). Scale bar=200 μm. (D) Magnified view of
Fig. 1C showing an egg with filaments (empty arrow). Scale bar=60 μm.
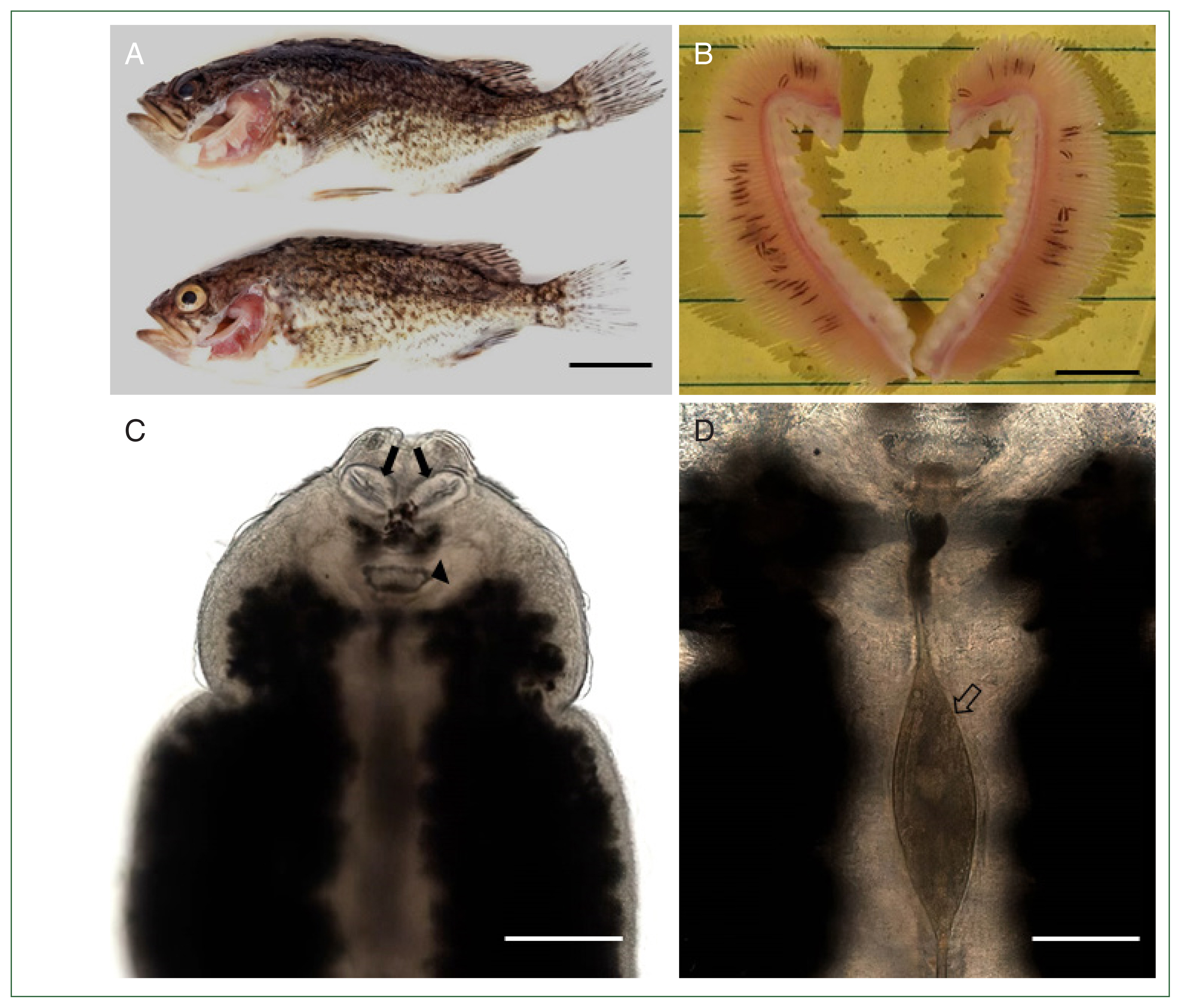
Fig. 2Scanning electron microscopic views of
Microcotyle sebastis. (A) Whole body with a characteristic opisthaptor possessing numerous clamps (white arrow). Scale bar=200 μm. (B) Anterior part with a genital atrium (arrow) located below the buccal cavity. Scale bar=50 μm. Magnified view of the genital atrium in a rectangle. Scale bar=20 μm. (C) Egg filament (white arrow) extruded from the genital pore. Scale bar=50 μm. (D) Magnified view of
Fig. 2C. Scale bar=10 μm. (E) Clamps showing numerous micropits (arrows) or microdepressions (empty arrows). Scale bar=20 μm. (F) The clamp consisting of lateral sclerites (arrows) and ventral mid-long (empty arrow) and dorsal mid-short (arrow head) sclerites. Scale bar=20 μm.
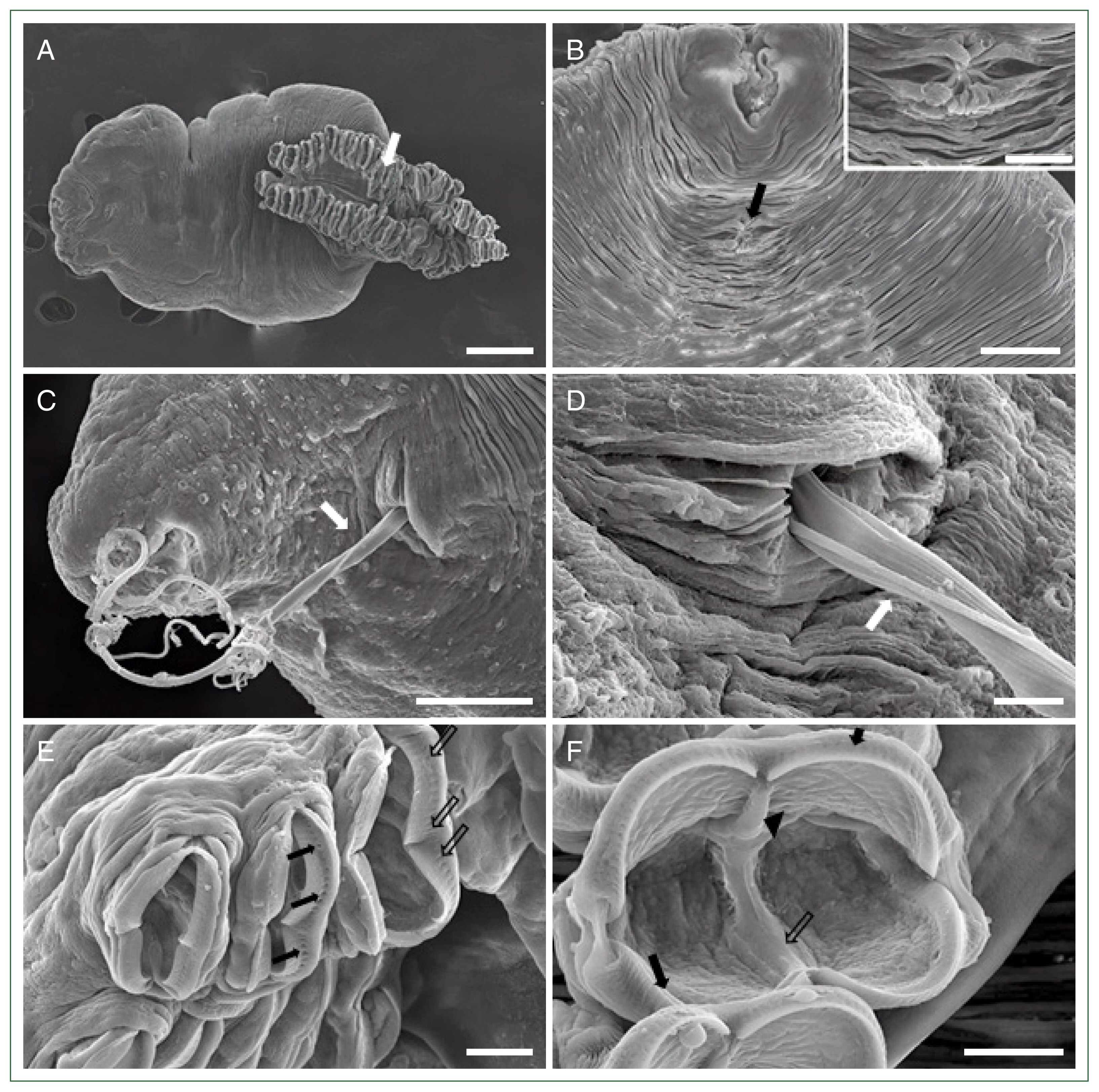
Fig. 3Scanning electron microscopic views of the gills of the Korean rockfish infested with
Microcotyle sebastis. (A) Lateral view. Scale bar=500 μm. (B) Dorsal view showing clamps (a and b) and scratch (b) in rectangles. Scale bar=100 μm. (C) Magnified view of clamps (arrows) in the rectangle (a) of
Fig. 3B, sucking the gill lamellae. Scale bar=20 μm. (D) Magnified view of the rectangle (b) of
Fig. 3B, showing the wound (arrow heads) in the gill lamellae by the sclerites. Scale bar=20 μm.

Fig. 4Histopathological findings of the gill filaments of the Korean rockfish infested with
M. sebastis. (A) The distal parts of gill lamellae are grasped by the clamps of the opisthaptor. Scale bar=500 μm. (B) Magnified view of the rectangle of
Fig. 4A. Distal parts of the lamellae were detached (arrows) and destroyed (empty arrow) by sclerites (red circle). Scale bar=60 μm.
References
- 1. Kearn GC. Evolutionary expansion of the monogenean. Int J Parasitol 1994;24(8):1227-1271.
https://doi.org/10.1016/0020-7519(94)90193-7
- 2. Reed P, Francis-Floyd R, Klinger R, Petty D. Monogenean parasites of fish: IFAS University of Florida FA28/FA033. EDIS 2012;1-10.
https://doi.org/10.32473/edis-fa033-2012
- 3. Li W, Yang B, Cheng J, Zou H, Li M, et al. Seasonal dynamics of Dactylogyrus species (Monogenea: Dactylogyridae) on wild and farmed goldfish (Carassius auratus): implication for prevention of dactylogyriasis. Aquac Rep 2022;26:101327.
https://doi.org/10.1016/j.aqrep.2022.101327
- 4. Tavares-Dias M, Silva LMA, Oliveira MSB. Geographic range, distribution patterns and interactions of Monogenea Van Beneden 1858, with species of native host freshwater fishes from Brazil. Rev Bras Parasitol Vet 2022;31(3):e005722.
https://doi.org/10.1590/S1984-29612022048
- 5. Kim KH, Choi ES. Treatment of Microcotyle sebastis (Monogenea) on the gills of cultured rockfish (Sebastes schelegeli) with oral administration of mebendazole and bithionol. Aquaculture 1998;167(1–2):115-121.
https://doi.org/10.1016/S0044-8486(98)00300-7
- 6. Kim KH, Cho JB. Treatment of Microcotyle sebastis (Monogenea: Polyopisthocotylea) infestation with praziquantel in an experimental cage simulating commercial rockfish Sebastes schlegeli culture conditions. Dis Aquat Organ 2000;40(3):229-231.
https://doi.org/10.3354/dao040229
- 7. Grano-Maldonado MI, Rodríguez-Santiago MA, García-Vargas F, Nieves-Soto M, Soares F. An emerging infection caused by Gyrodactylus cichlidarum Paperna, 1968 (Monogenea: Gyrodactylidae) associated with massive mortality on farmed tilapia Oreochromis niloticus (L.) on the Mexican pacific coast. Lat Am J Aquat Res 2018;46(5):961-968.
https://doi.org/10.3856/vol46-issue5-fulltext-9
- 8. Bai SC, Okorie OE. Korean rockfish (Sebastes schlegeli) production in Korea. Glob Aquac Advocate 2009;1-4.
- 9. Kang GH, Cha SJ. Monitoring of pathogens detected in cultured fishes of Gyeongnam in 2018. Korean J Fish Aquat Sci 2019;52(5):539-546. (in Korean). https://doi.org/10.5657/KFAS.2019.0539
- 10. Rubio-Godoy M. Fish host-monogenean parasite interactions, with special reference to Polyopisthocotylea. In Terrazas LI ed, Advances in the Immunobiology of Parasitic Diseases. Research Signpost; Kerala, India. 2007, pp 91-109.
- 11. Yoon GH, Shinn A, Sommerville C, Jo JY. Seasonality and the microhabitat of Microcotyle sebastis Goto 1894, a monogenean gill parasite of farmed rockfish, Sebastes schlegeli Hilgendorf 1880. J Aquacult 1998;10(4):387-394. (in Korean).
- 12. Song JY, Kim KY, Choi SW. Occurrence and molecular identification of Microcotyle sebastis isolated from fish farms of the Korean rockfish, Sebastes schlegelii. Korean J Parasitol 2021;59(1):89-95.
https://doi.org/10.3347/kjp.2021.59.1.89
- 13. Goto S. Studies on the ectoparasitic trematodes of Japan. J Coll Sci Imp Univ Tokyo 1894;8:1-273.
- 14. Beverley-Burton M. 1984, Monogenea and turbellaria. In Margolis L, Kabata Z eds, Guide to the Parasites of Fishes of Canada. Part I Canadian special publications in fisheries and aquatic sciences 74. Ottawa, Canada. 1984, pp 170-174.
- 15. Bonham K, Guberlet JE. Notes on Microcotyle sebastis Goto from Puget Sound. J Parasitol 1937;23(3):281-290.
https://doi.org/10.2307/3272417
- 16. Montero FE, Crespo S, Padrós F, De La Gándara F, García A, et al. Effects of the gill parasite Zeuxapta seriolae (Monogenea: Heteraxinidae) on the amberjack Seriola dumerili Risso (Teleostei: Carangidae). Aquaculture 2004;232(1–4):153-163.
https://doi.org/10.1016/S0044-8486(03)00536-2
- 17. Bullard SA, Frasca S Jr, Benz GW. Gill lesions associated with Erpocotyle tiburonis (Monogenea: Hexabothriidae) on wild and aquarium-held bonnethead sharks (Sphyrna tiburo). J Parasitol 2001;87(5):972-977.
https://doi.org/10.1645/0022-3395(2001)087[0972:GLAWET]2.0.CO;2
- 18. Kim KH, Hwang YJ, Cho JB, Park SI. Immunization of cultured juvenile rockfish Sebastes schlegeli against Microcotyle sebastis (Monogenea). Dis Aquat Organ 2000;40:29-32.
https://doi.org/10.3354/dao040029
- 19. Thoney DA. Post-larval growth of Microcotyle sebastis (Platyhelminthes: Monogenea), a gill parasite of the black rockfish. Trans Am Microsc Soc 1986;105(2):170-181.
https://doi.org/10.2307/3226389