Trichomonas vaginalis and trichomoniasis in the Republic of Korea
Article information
Abstract
Vaginal trichomoniasis, caused by Trichomonas vaginalis, is the most common sexually transmitted disease. More than 170 million people worldwide are annually infected by this protozoan. In the Republic of Korea, 10.4% of women complaining of vaginal symptoms and signs were found to be infected with T. vaginalis. However, despite its high prevalence, the pathogenesis of T. vaginalis infection has not been clearly characterized although neutrophil infiltration is considered to be primarily responsible for the cytologic changes associated with this infection. We hypothesized that trichomonads in the vagina sometime after an acute infection secrete proteins like excretory-secretory product that have a chemotactic effect on neutrophils, and that these neutrophils are further stimulated by T. vaginalis to produce chemokines like IL-8 and GRO-α, which further promote neutrophil recruitment and chemotaxis. Thus, neutrophil accumulation is believed to maintain or aggravate inflammation. However, enhanced neutrophil apoptosis induced by live T. vaginalis could contribute to resolution of inflammation. Macrophages may constitute an important component of host defense against T. vaginalis infection. For example, mouse macrophages alone and those activated by lymphokines or nitric oxide are known to be involved in the extracellular killing of T. vaginalis. In the host, T. vaginalis uses a capping phenomenon to cleave host immunoglobulins with proteinases and thus escape from host immune responses. Recently, we developed a highly sensitive and specific diagnostic polymerase chain reaction (PCR) technique using primers based on a repetitive sequence cloned from T. vaginalis (TV-E650), and found that the method enables the detection of T. vaginalis at concentrations as low as 1 cell per PCR mixture.
INTRODUCTION
Trichomonas vaginalis is a flagellate protozoan that parasitizes the human vagina, prostate gland, and urethra. Trichomoniasis caused by T. vaginalis is generally believed to be the most common sexually transmitted disease, and more than 170 million people are believed to be infected annually (WHO, 1995a). Two to 3 million symptomatic infections per year are believed to occur among sexually active women in the United States (Wolner-Hanssen et al., 1989). In the Republic of Korea, 10.4% of women complaining of vaginal symptoms and signs have been reported to be infected with T. vaginalis (Ryu et al., 1999).
In pregnant women, trichomonads are implicated in premature membrane rupture and delivery, and in the delivery of low-birth-weight infants (Minkoff et al., 1984; Soper et al., 1990). In addition, trichomoniasis has been implicated as a risk factor of human immunodeficiency virus transmission (Laga et al., 1993).
The present review covers overall studies on T. vaginalis published in the Republic of Korea and other related papers on vaginal trichomoniasis. The main content of this review is divided into 6 topics, namely, morphology, epidemiology, pathogenesis, host-parasite relationships, diagnosis, and chemotherapy.
MORPHOLOGY
Morphologically T. vaginalis has only the trophozoite stage, resembling other trichomonads. The trophozoites are 23 to 39 µm long (including a 8-13 µm body length and 8-15 µm flagella length) and 5 to 8 µm wide. There are 4 anterior flagella, and a fifth flagellum is incorporated within its undulating membrane. The axostyle (3-14 µm in length) is usually extremely obvious, and the undulating membrane extends about 2/3 of the distance to the posterior end of the body, with no free flagellum (Fig. 1A). Nuclear chromatin is uniformly distributed, and there are a large number of hydrogenosomes (formerly called siderophil granules) that are particularly evident around the axostyle. T. vaginalis does not have mitochondria in its cytoplasm. Instead, hydrogenosomes serve as mitochondria, and have been found to be important metabolic features that participate in energy production and drug activation. These hydrogenosomes are round to oval in shape and were so named because they produce molecular hydrogen as end product of metabolism (Müller, 1980) (Fig. 1B).
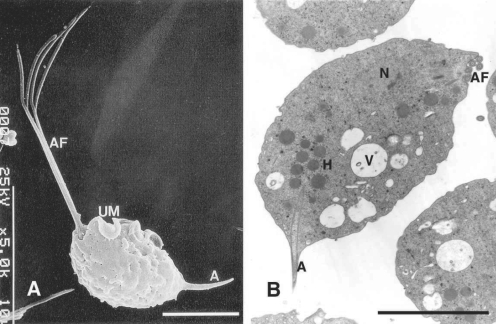
A. Normal trophozoites of T. vaginalis showing 4 anterior flagella (AF), an undulating membrane (UM) with posterior flagellum, and an axostyle (A). B. This transmission electron microscope photograph illustrates a nucleus (N), hydrogenosomes (H), anterior flagella, many vacuoles (V) and an axostyle. Bar = 5 µm (Kim and Ryu, 2001; Min et al., 1994)
Using scanning electron microscopy the authors compared the sizes of trophozoites cultivated in trypticase-yeast extract-maltose (TYM) medium with those of trophozoites freshly isolated from vaginitis patients, and it was found that the lengths of the axostyles and flagella of trophozoites freshly isolated from trichomoniasis patients were significantly longer (data not shown). It is believed that trophozoite shape and length may be changed by environmental conditions, i.e., in vivo in the vagina and in vitro. The authors have previously suggested that the schematic morphologies of T. vaginalis illustrated in many parasitology textbooks should be amended to correspond to those of trophozoites isolated from vaginitis patients.
EPIDEMIOLOGY
Trichomonas vaginalis was first described by Donne in 1836 and has long been regarded as a harmless commensal of the human vagina. However, in 1916 Hohne used the term "trichomoniasis" to describe the condition wherein the vagina contained many of these flagellates. Subsequently, the concept of T. vaginalis as a primary pathogenic parasite was adopted, and Trussell and Plass (1940) concluded that T. vaginalis produced vaginitis in 9 of 20 women by culturing T. vaginalis. In addition, it is known that T. vaginalis commonly causes vaginitis, and perhaps cervicitis, in women, and urethritis in both sexes.
The prevalence of infection varies according to investigators and subjects. The first record of T. vaginalis in the Republic of Korea was reported by Na (1947) at an Annual Meeting of the Korean Society of Obstetrics and Gynecology. It was reported that the overall rate of infection among patients that visited an obstetrics and gynecology clinic in Seoul was 17.3%, and thus, it was concluded that vaginal trichomoniasis is not a rare disease in the Republic of Korea. Later, studies on trichomonad vaginitis by Shin and Kim (1957), revealed a high prevalence (42%) of this flagellate among Korean women, and drew attention to the significance of T. vaginalis as a major cause of vaginitis. Soh et al. (1961), Kim (1962) and Chae (1965) reported the prevalence of 35.8% (410/1,146) among outpatients of Severance Hospital, 20.8% (54/260) among Seoul National University Hospital outpatients, and 24.1% (40/166) among outpatients at the National Masan Hospital in Gyeongsangnam-do. Chung et al. (1969) found a slightly higher prevalence of 29.5% (71/241) among prostitutes at Yeosu-si, Jeollanam-do and Gunsan-si, Jeollabuk-do.
In the 1970's, 2 reports indicated that the prevalenceof T. vaginalis remained high, i.e., 21.9% (139/638) in Daegu (Chang and Choi, 1976), and 23.3% (93/400) among 400 women from Hongseong-gun and Cheongyang-gun in Chungcheongnam-do (Ro, 1977), although a lower prevalence rate (9.3%, 600/6,457) was recorded at this time among 6,459 outpatients at Chungnam National University Hospital (Lee, 1977).
After the 1980's, prevalences fell to 10% or less. Kang et al. (1989) and Choi et al. (1996) reported prevalences of 7.8% (14/180) and 7.6% (478/6262) among obstetrics and gynecology out-patients in Daejeon and Gangwon-do, respectively, and Ryu et al. (1999) reported 17 infections (10.4%) among 177 patients with a vaginal discharge and 6 infections (2.4%) were recorded among 249 who visited for a regular cervical Papanicolaou smear with no vaginal symptoms. These findings indicate that the T. vaginalis infection rate among women with vaginal symptoms and signs may remain relatively high.
Prostitutes and mistresses are expected to show higher infection rates than house wives. Epidemiological studies of T. vaginalis in Yeosu-si and Gunsan-si areas indicated a high incidence of 29.5% (71/241) among prostitutes examined (Chung et al., 1969). Moreover, foreigner's mistresses and prostitutes in Daegu showed a higher prevalence of 35.1% (66/188) and 53.2% (164/308) compared to 21.9% (139/634) among house wives (Chang and Choi, 1976). Moreover, Kang et al. (1989) reported prevalences of 18.9% among 217 prostitutes and of 7.8% among 180 obstetrics and gynecology out-patients in the Daejeon area.
Peak vaginal trichomoniasis incidence occurs between the ages of 16 and 35, the period of greatest sexual activity (Beaver et al., 1984). Similarly, Korean investigators observed a similar tendency, e.g., Kang et al. (1989) and Lee (1977) found that 20-29 year olds were most affected, and Chang and Choi (1976) and Ro (1977) found that 30-34 year olds were most affected among obstetrics and gynecology out-patients.
Whereas epidemiological, clinical and therapeutic studies on T. vaginalis among Korean women have been reported by many investigators, relatively few studies have been published concerning this parasite in men. Chu et al. (1974) and Chu et al. (1990) surveyed trichomoniasis among 9,617 male in- and out-patients at Kyunghee Medical Center, Seoul, and among 10,144 out-patients at Cheil hospital in Jochiwon-eup, Chungcheongnam-do, using urine wet smears and acridine orange, hematoxylin stained smears. In both studies it was found that the prevalence of trichomoniasis in men was low, i.e., 0.5% and 1.1%, respectively. A survey of trichomoniasis prevalence among Korean military personnel without noticeable symptoms showed higher prevalences of 3.4% (33/977; Joo and Choi, 1980) and 3.3% (16/480; Kim, 1977) by prostate culture and wet smear. Moreover, for military personnel with lower urinary tract symptoms, the prevalence of T. vaginalis was found to be higher at 7.5% (9/120) (Kim, 1977). In men, infection sites included the urethra in 68%, prostate in 40%, and bladder and epididymis in 4%. The most frequent subjective symptoms were an itching sensation in the urethra (16%), perineal discomfort (12%), dysuria (12%) and a urethral discharge (8%). These results demonstrated that T. vaginalis was an important disease in Korean military personnel, and that eradication is possible given extensive public health education and the administration of specific therapeutic agents. Unfortunately, no further reports have been issued on the prevalence of T. vaginalis among military personnel.
However, T. vaginalis has been isolated from 1 to 68% of men with non-gonococcal urethritis (NGU) in epidemiologic studies (Lee and Joo, 1961; Krieger, 1982), and the number of NGU cases is increasing more rapidly than gonorrhea in developed nations, and in some developing countries, especially in the Republic of Korea. Jeong et al. (1993) reported a 6.7% (14/208) positive reaction rate to IgG antibody among 208 NGU patients using enzyme-linked immunosorbent assay (ELISA). Further study is required to establish the prevalence of T. vaginalis in men, including symptomatic patients and military personnel, using more sensitive PCR-like diagnostic methods as a means of improving public health control.
Transmission mode possibilities other than sexual transmission
The authors tested the possibility of T. vaginalis transmission by means other than sexual contact. Trophozoites were suspended in various environmental conditions, and their survival rates were measured using a hemocytometer after trypan blue staining. In addition, the drying times of vaginal secretions exposed at different temperatures (4, 26, and 30℃) were observed. T. vaginalis survival rates were found to reduce as tap water temperature increased. For example, fewer than 10% trophozoites survived 30 min of exposure at 4℃ or 15 min at 26℃. Moreover, hot water at > 45℃ killed trichomonads within 5 min or so, as did swimming pool or cleaning solution (detergent, bleaching solution). When trophozoites were placed in the urine samples of 6 healthy individuals, survival rates were less than 10% after 24 hr of exposure, but with notable individual variations. Vaginal secretions were also placed on glass slides and left to dry at 4℃ in a refrigerator, and at 26℃ and 30℃ in incubators. It was found to take 70 min, 44 min, and 26 min, respectively, to dry vaginal secretions under these conditions. Moreover, it should be noted that trichomonad survival was not affected until complete dryness was achieved (Table 1). These results indicate that T. vaginalis may be transmitted by contact with contaminated toilet facilities, but that this type of transmission is probably uncommon (Ryu et al., 2002).

Drying time of vaginal secretions at different temperatures (Ryu et al., 2002)
Prior to the 1970's, many women used a chamber pot; 'yo-gang' in Hangul (the Korean language), because toilets were generally outdoors or located at some distance from bedrooms. In addition, a number of family members slept in 1 bedroom and shared a yo-gang, and therefore, yo-gangs contaminated with T. vaginalis may have constituted a source of infection.
PATHOGENESIS
The most common complaint associated with vaginal trichomoniasis infection is a vaginal discharge. This discharge is frequently profuse and is often associated with burning, itching, or chafing. When viewed through a speculum, the vaginal mucosa is sometimes diffusely hyperemic, with bright red punctate lesions, although sometimes it is only patchily hyperemic and not infrequently normal in appearance. The frequency of urination and dysuria are the commonest associated symptoms, and urethral involvement is found in a large proportion of cases (Markell et al., 1999).
Korean gynecologists have reported similar symptoms and signs. Trichomoniasis patients show various symptoms and/or signs of vaginitis that included vaginal discharge (64.7%), pruritus (35.3%), erythema, vaginal wall edema (17.6%), and dyspareunia (5.9%) (Ryu et al., 1999). Lee (1977) also observed leucorrhea (80.5%), itching (34.5%), a foul odor (18.7%), low abdominal discomfort (17.7%), dysuria (16.8%), vulvar pain (9.3%), dyspareunia (6.8%), and bloody discharge (6%).
Factors involved in the pathogenesis of T. vaginalis
The factors considered to be involved in the pathogenicity of T. vaginalis include; the ability of trichomonads to adhere to vaginal epithelial cells (VECs), the cytotoxic effect of the pathogen on host cells, trichomonad proteinase activity, and the ability of trichomonads to produce subcutaneous abscess lesions in mice (Alderete and Pearlman, 1984; Alderete and Garza, 1985; Alderete et al., 1995; Krieger et al., 1985).
Kim et al. (1987) used a cytotoxicity test in order to determine the pathogenicity of T. vaginalis. The cytotoxicities of 12 isolates of T. vaginalis on HeLa, Hep-2, and Vero cell lines were measured using the crystal violet staining method. These results were then compared with the subcutaneous abscess sizes of mice. At a parasite to HeLa cell ratio of 1 : 2 and after an incubation of 12 hr, highly pathogenic isolates killed 80% of cells, whereas mildly pathogenic isolates killed 40 to 80%, and isolates with low pathogenicitykilled less than 40%. These results suggest that the in vitro cytotoxic effect of T. vaginalis on HeLa cells might provide a useful tool for distinguishing pathogenic isolates of T. vaginalis.
Shim et al. (1993) measured the proteinase activities of T. vaginalis isolates and compared these with their pathogenicities. In this study, 10 axenic isolates of T. vaginalis were subcutaneously injected into BALB/c mice in order to assess their pathogenicities. All isolates had neutral and acid proteinase activities in their lysates and in culture media, but the specific activities of both proteinase types in the highly pathogenic group were significantly greater than in the mildly pathogenic group (P < 0.05). An SDS-PAGE system, in which electrophoretic gels contained 0.4% gelatin as substrate, showed 5 different banding patterns for trichomonal proteinases, and these patterns were found to be closely related with the pathogenicities of T. vaginalis isolates. Inhibitor assays indicated that all 5 bands were cysteine proteinases. In addition, the cytotoxic effects of lysates of T. vaginalis on a Chinese hamster ovarian (CHO) cell line were also found to be related to pathogenicity of T. vaginalis isolate, and in general cytotoxic effects were lower for lysates treated with cysteine proteinase inhibitors than for control lysates. In summary these results indicate that the proteinases of T. vaginalis, which show 5 electrophoretic banding patterns, are associated with the pathogenicities and cytotoxicities of T. vaginalis isolates.
In terms of the pathogenesis of T. vaginalis, parasite attachment to host cells is a prerequisite for the establishment of infection, as the organism must overcome constant vaginal secretions. Many studies on the specificity of the adherence of T. vaginalis to VECs have demonstrated that adherence is a multifactor process, in which microtubules, microfilaments, 4 adhesins and cysteine proteinase participate (Alderete et al., 1995; Krieger et al., 1985; Mendoza-Lopeza et al., 2000). Kim and Ryu (2001) observed under a scanning electron microscope morphologic changes in T. vaginalis after contact with human VECs. In one experiment, the authors mixed VECs of normal women with T. vaginalis and incubated for 30 min. It was found that parasitic bodies became elongated with pseudopodia or flattened in an ameboid fashion, and that they showed strong adherence to VECs, unlike trichomonads exposed to HeLa cells (Figs. 2A-C).
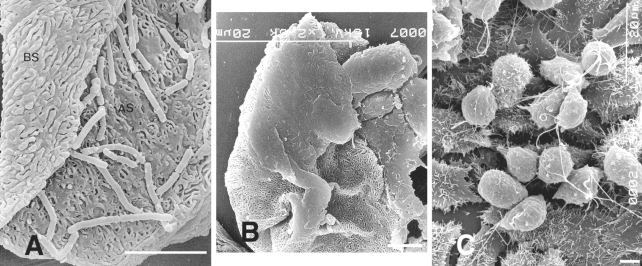
A. Both surfaces, apical (AS) and basal (BS) surfaces, of human vaginal epithelial cells (HVECs) are observed. The microridges of the apical surface frequently surround a pit or a hole. B. Flattened trichomonads stick to HVECs. C. Many trichomonads adhere to the HeLa cell monolayer. The majority of parasites that attach to HeLa cells retain the pear-like shape. Bar = 5 µm. (Kim and Ryu, 2001)
Adherence is also important for interleukin-8 (IL-8) production by neutrophils, when live trichomonads were pretreated with various inhibitors of proteinases, microtubule, microfilament, or adhesin (all of which are known to participate in the adherence of T. vaginalis to VECs), IL-8 production was found to be significantly reduced versus untreated controls (Ryu et al., 2004).
HOST-PARASITE RELATIONSHIPS
The pathogenesis of T. vaginalis infection has not been fully elucidated although neutrophil infiltration is considered to be primarily responsible for cytologic changes (Fouts and Kraus, 1993; Graves and Gardner, 1993). Moreover, little is known about how neutrophils accumulate or mediate initial inflammatory response after acute T. vaginalis infection. Nevertheless, it is generally believed that chemoattractants generated at reaction sites play important roles. The chemoattractants proposed to be involved in inflammatory response to T. vaginalis include leukotriene B4 and IL-8, both of which are found in the vaginal discharges of symptomatic trichomoniasis patients (Shaio et al., 1994; Shaio and Lin, 1995).
Park et al. (1984) investigated the chemotactic patterns elicited by autologous and heterologous sera, and by trichomonad secretions on leukocytes from normal and immunized rabbits. Blind well Boyden chambers were used to measure leukocyte chemotaxis. For transfilter cell movement studies, lower membrane surfaces (Millipore pore size; 5 µm) were examined using a 40 × objective, and counts were made of cells that had traversed membranes to lower membrane surfaces in high power fields. The above experiment demonstrated that the metabolic end products of T. vaginalis and serum complement may act as leukocyte chemotactic factors.
Ryu et al. (2004) measured levels of IL-8, a strong chemoattractant for neutrophils, and found that when human neutrophils were stimulated with live trophozoites, T. vaginalis lysate, or T. vaginalis excretory-secretory (ES) product, that live trichomonads induced higher levels of IL-8 production (Fig. 3). Moreover, when live trichomonads were pretreated with various inhibitors of proteinase, microtubule, microfilament, or adhesin, which are all known to participate in the adherence of T. vaginalis to VECs, IL-8 production was significantly reduced compared with untreated controls. Furthermore, an NF-κB inhibitor (pyrrolidine dithiocarbamate), a MAP kinase (MEK) inhibitor (PD98059), and a p38 MAP kinase inhibitor (SB203580) significantly suppressed IL-8 synthesis in neutrophils. These results suggest that live T. vaginalis, particularly when adherence is retained, can induce IL-8 production in neutrophils, and that this action may be mediated through NF-κB and MAP kinase signaling pathways (Figs. 4A, 4B). In other words, T. vaginalis-induced neutrophil recruitment may be mediated via the IL-8 expressed by neutrophils in response to activation by live T. vaginalis.
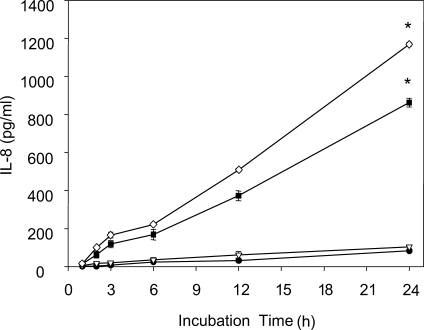
IL-8 production by human neutrophils treated with live T. vaginalis. Neutrophils (2 × 105/well) were stimulated with 1 × 104 (▿), 2 × 104 (■) or 4 × 104 (◇) T. vaginalis or medium alone (●) for 1 to 24 h at 37℃. Culture supernatants were collected at the indicated times, and secreted IL-8 levels were measured by ELISA. Data are presented as means ± SE of 3 experiments performed in triplicate. * P < 0.05. (Ryu et al., 2004)
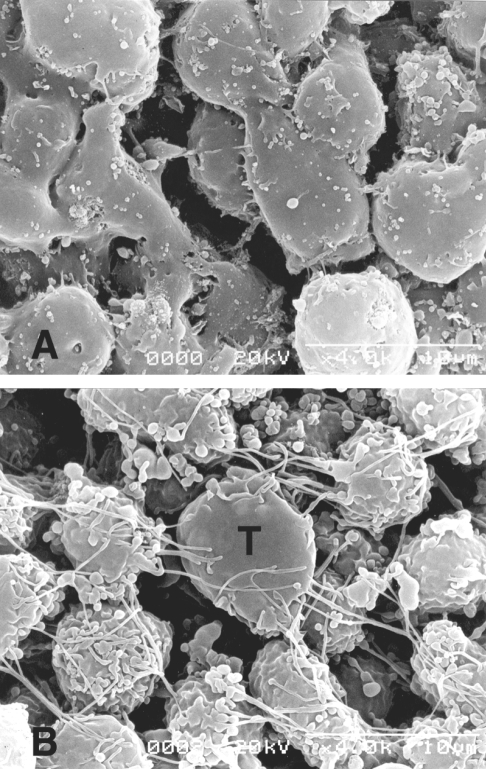
Scanning electron micrographs of neutrophils. Neutrophils (2 × 106) and T. vaginalis (2 × 105) were co-incubated for 24 h at 37℃. A. Neutrophils alone; the few microvilli present were small spherical extensions of the cell membrane, though some were located on short processes or stalks. B. When the neutrophils were interacted with T. vaginalis at a ratio of 10 : 1, several activated neutrophils surrounded one trophozoite, and extended many filopodia toward the trichomonad (T). The plasma membranes of stimulated neutrophils contained irregular ridges and small ruffles, which contrasted with the smooth plasma membranes of normal neutrophils. (Ryu et al., 2004)
Based on the above-described study and several earlier studies, we hypothesize that many trichomonads in the vagina, after acute T. vaginalis infection, secrete proteins, including ES product, and that these have a chemotactic effect on neutrophils. In addition, it appears that these neutrophils can be further stimulated by T. vaginalis to produce chemokines, such as, IL-8 and GRO-α, which may subsequently further promote infiltration and the recruitment of neutrophils by chemotaxis at reaction sites. Moreover, the involvement of vaginal epithelial cells during early infection might promote IL-8 production, and finally, neutrophil accumulation is believed to cause continued inflammation and/or aggravate vaginal inflammation.
Kang et al. (2006) showed that T. vaginalis increases human neutrophil apoptosis, and that this contributes to the resolution of inflammation. The authors of the present study examined whether T. vaginalis can induce neutrophil apoptosis, and whether caspase-3 and Bcl-2 family members are involved in this process. Accordingly, human neutrophils were incubated with live T. vaginalis and apoptosis was evaluated by Giemsa, annexin V-PI, and DiOC6 staining. Neutrophil apoptosis was found to be significantly higher for cells incubated with T. vaginalis, and when trichomonads were pretreated with mAb to AP65 (adhesin protein), and when trophozoites were separated from neutrophils using a Transwell chamber, neutrophil apoptosis was significantly reduced. Moreover, caspase-3 activation was evident in neutrophils undergoing spontaneous apoptosis, and this activation was markedly enhanced during T. vaginalis-induced apoptosis. In addition, the inhibition of caspase-3 effectively reduced T. vaginalis-induced apoptosis, and trichomonad-induced apoptosis was also associated with the down-regulation of the neutrophil antiapoptotic protein, Mcl-1. These results indicate that T. vaginalis alters Mcl-1 expression and caspase-3 activation, and thereby, induces the apoptosis of human neutrophils, which strongly suggests that increased neutrophil apoptosis by T. vaginalis contributes to the resolution of inflammation. The authors are currently attempting to determine whether macrophages can phagocytose the apoptotic neutrophils induced by T. vaginalis.
Macrophages have an important role in host innate immunity. Ryu et al. (1990) reported that mouse peritoneal macrophages have a spontaneously cytotoxic effect on T. vaginalis, and that lymphokines (produced by phytohemagglutinin-stimulated spleen cells) increase this cytotoxicity by activating macrophages to kill T. vaginalis. Yoon et al. (1991) undertook to identify cytokines with cytotoxic effects on T. vaginalis, and found that the addition of rIL-2 or rIFN-γ enhanced the cytotoxic effect of macrophages, whereas rIL-4 inhibited this effect.
Chang et al. (2004b) reported that T. vaginalis inhibits proinflammatory cytokine production in macrophages (murine monocyte/macrophage cell line, RAW264.7) by suppressing NF-κB activation, which may have a role in the evasion of host immunity. In addition, T. vaginalis was found to induce apoptotic cell death via a Bcl-xL-dependent pathway and via the phosphorylation of p38 MAPK in RAW264.7 macrophages (Chang et al., 2004a, 2006).
Nitric oxide (NO) has been reported to play an important role in the host defense mechanism elicited by macrophages against T. vaginalis. Park et al. (1997) found that NO is involved in the extracellular killing of T. vaginalis by mouse peritoneal macrophages and that RAW264.7 cells are activated by LPS or rIFN-γ. Moreover, NO inhibitors such as NG-monomethyl-L-arginine (L-NMMA), NG-nitro-L-arginine methyl ester (NAME), and arginase inhibited the cytotoxic effects on T. vaginalis and nitrite production by activated mouse peritoneal macrophages and RAW264.7 cells. Moreover, the addition of excess L-arginine competitively restored the trichomonacidal activities of macrophages. These results suggest that NO plays an important role in the host defense mechanism induced by macrophages against T. vaginalis.
In addition, a cell-mediated response was observed in mice infected with T. vaginalis (Shin et al., 1990). In this study trichomonads were inoculated subcutaneously (1 × 106/animal) into the backs of BALB/c mice. Following injection, delayed type hypersensitivity (DTH) response was observed in footpads, and blastogenic responses of mouse spleen cells were observed by [3H]-thymidine incorporation, 1, 4 and 7 weeks after inoculation. Blastogenic responses of splenocytes to T. vaginalis lysate and ES products, and delayed type hypersensitivity responses were markedly increased in infected mice compared with control mice at 7 weeks after infection. Moreover, serum antibody titers in mice infected with T. vaginalis increased continuously from 1 to 8 weeks post-inoculation. These results suggest that humoral immunity and cell-mediated immunity are both involved in mice experimentally inoculated with T. vaginalis.
Factors related to the virulence of T. vaginalis
Iron is essentially required by many pathogens, including T. vaginalis, for growth. In fact, iron is required by all eukaryotic organisms, because it is essentially required for many metabolic processes, including oxygen and electron transport, and DNA synthesis (Conrad et al., 1999). Ryu et al. (2001a) evaluated the role of iron with respect to the virulence of T. vaginalis in mice. Iron-supplemented and iron-depleted Diamond's trypticase-yeast extract-maltose (TYM) media were prepared by adding 360 µM ferrous sulfate or 100 µM 2,2'-dipyridyl. Trophozoites cultivated in normal TYM and iron-supplemented TYM were found to produce subcutaneous abscesses, but trichomonads cultivated in iron-deficient TYM medium had no pathologic effect (Table 2). In addition, iron affected the adherence and the cytotoxic effect of trichomonads on HeLa cells, and these were significantly reduced when trophozoites were grown in iron-deficient TYM medium. These findings suggest that under iron depleted conditions, such as, those induced by 2,2'-dipyridyl, that the virulence of T. vaginalis is reduced.
Comparison of abscesses produced by Trichomonas vaginalis cultivated in iron-deficient TYM, normal TYM, and iron-supplemented TYM media (Ryu et al., 2001)
In trichomoniasis, the onset of vulvar and vaginal pruritus and discharge are usually acute and occurs during, or immediately after, menstruation, which is probably due to increased vaginal acidity (Beaver et al., 1984). T. vaginalis probably obtains iron from lactoferrin in vaginal mucosa, or from erythrocytes (Peterson and Alderete, 1984; Lehker et al., 1990). Moreover, the concentration of lactoferrin in vaginal mucosa is highest after menstruation and lowest immediately prior to menstruation (Cohen et al., 1987). Therefore, the most favorable period in terms of iron availability from lactoferrin for the growth of T. vaginalis is immediately after menstruation. Moreover, such conditions probably promote trichomonad pathogenicity by increasing cytoadherence, cytotoxicity, and proteinase activity. Therefore, the authors suggest that the aggravation of symptoms and signs caused by T. vaginalis after menstruation is probably related to an iron-rich environment and increased vaginal acidity (Ryu et al., 2001a).
Immune escape mechanisms of T. vaginalis
Escape mechanisms from the host's immune system have been well documented for various protozoan parasites. These include; the use of a location relatively shielded from immune attack; the generation of surface antigen variations, and their shedding and disguising; and the subversion of host immune responsiveness (Cohen, 1982). The ability of a pathogen to shed and renew surface antigens clearly provides it with the potential to evade specific immune effector process. In trichomoniasis specific antibodies in serum and vaginal secretions have been isolated and well characterized (Alderete, 1984), although biological modifications of the parasite's membrane after antibody exposure are not understood. Ryu et al. (1992) described the capping phenomenon, that enables a pathogen to escape immune response by removing antibodies bound to antigens on the parasite's surface membrane. In this study, an isolated strain of T. vaginalis was incubated with rabbit anti-serum at 4℃, 25℃, or 37℃ for 30 min, 1 hr, or 2 hr, and then treated with fluorescein labeled anti-rabbit IgG (Fig. 5A, 5B). Cell-capping was clearly observed under a fluorescent microscope, and was found to be temperature dependent. Moreover, sodium azide, a metabolic inhibitor, inhibited T. vaginalis capping, whereas colchicine and vincristine, microtubule synthesis inhibitors, did not affect cap formation. These results suggest that antibody-induced membrane antigen modulation by T. vaginalis may resist host immune responses.
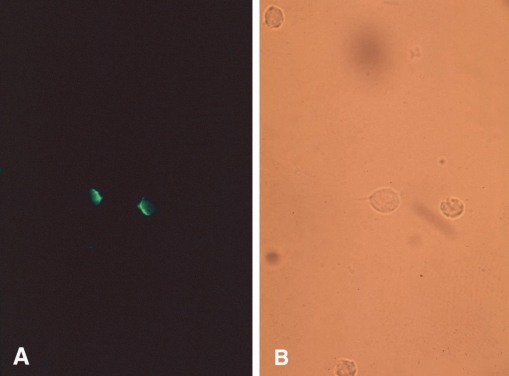
T. vaginalis incubated with specific antibody and FITC-labeled anti-rabbit IgG at 37℃ for 2 hrs. The same field is shown under UV illumination (A) and visible light (B). Most cells fluoresced at poles (capping). (Ryu et al., 1992)
Live T. vaginalis also degrade human secretory IgA, and serum IgA and IgG, and these degradations are increased by increasing incubation times and trichomonad numbers, respectively. In addition, a 60kDa cysteine proteinase of T. vaginalis, purified by affinity chromatography and gel filtration, degraded IgA and IgG. However, cysteine and serine proteinase inhibitors, i.e., E-64, antipain, iodoacetic acid, iodoacetamide, and TLCK (Na-p-tosyl-L-lysiue chloromethylketone) reduced the cleavage of immunoglobulins. Min et al. (1997, 1998) suggested that the abilities of parasite proteinases to cleave host immunoglobulins provide a means of immune evasion mechanism in the host.
DIAGNOSIS
Direct wet mount examinations of vaginal secretion, which are widely used for T. vaginalis diagnosis, are rapid and economical. However, the sensitivity of this technique is not high. Yi et al. (1990) employed ELISA to detect serum anti-T. vaginalis IgG and IgM antibodies in 30 vaginal trichomoniasis patients and 30 non-infected healthy individuals. The sensitivity and specificity of ELISA using 1 T. vaginalis isolate for serum IgG and IgM antibody detection were 70% and 96.7%, respectively. To enhance ELISA sensitivity, Ryu et al. (2000) used a mixed lysate antigen of 6 isolates (KT4, KT11, KT12, KT18, CDC85 and IR78). The sensitivity of this ELISA was 95.0%, and the sensitivity of the lysate from KT4 isolate and mixed ES antigen from the 6 isolates were 86.4% and 76.3%, respectively. Specificities of ELISA based on the above 3 antigens (a mixed lysate antigen of 6 isolates, a lysate from the KT4 isolate and mixed ES antigen from the 6 isolates) were 93.3%, 96.3% and 92.0%, respectively. These findings suggest that ELISA based on the mixed lysates of 6 T. vaginalis isolates could be useful for the diagnosis of trichomoniasis.
Yoon et al. (1987) applied the indirect fluorescent antibody (IFA) test to the detection of serum IgG and IgM antibodies for T. vaginalis in 31 vaginal trichomoniasis and in 7 candidiasis cases and in 20 non-infected healthy women, using antigen prepared from an axenic culture of T. vaginalis isolated from a vulvovaginitis patient. The sensitivity and specificity of this IFA test for IgG antibody to trichomonad antigen were 87.1% and 100%, respectively, which suggests that this test could be used to detect anti-trichomonad IgG antibodies and as an immunodiagnostic method. Kim et al. (1983) compared the diagnostic efficacies of ELISA, indirect immunofluorescent antibody test (IFAT), and thin-layer immunoassay (TIA) for trichomoniasis. A significant correlation was observed between the antibody titers of trichomoniasis cases detected by ELISA and IFAT, again it was concluded that IFAT and ELISA could be usefully used to diagnose trichomoniasis.
Various methods have been used to diagnose trichomoniasis, such as, wet mount, culture, Papanicolaou smear, and serologic tests. Wet mount examinations are straightforward and rapid, but more than 103/ml of live protozoa are required for detection, and cultures require specialized medium and in total 2-5 days are required to make a diagnosis. The accurate interpretation of Papanicolaou smear results also requires a skilled observer. Moreover, the major limitation of serological tests for the detection of T. vaginalis by indirect immunofluorescence remains its lack of sensitivity and specificity.
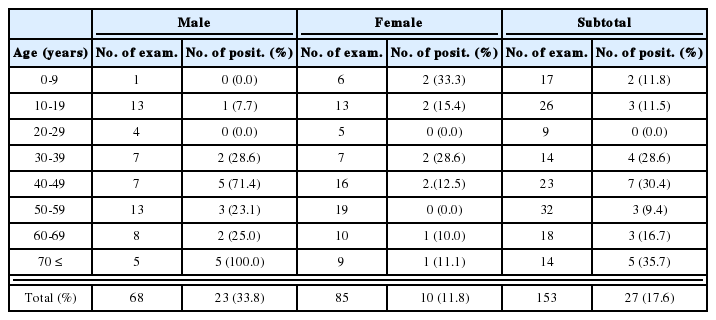
During recent years, molecular biological techniques have provided new approaches to the diagnosis of parasite infections. PCR permits the in vitro amplification of DNA fragments, and has introduced new possibilities for the diagnosis of numerous agents in addition to parasites. Ryu et al. (1999) developed a highly sensitive and specific PCR method using primers based on the repetitive sequence of T. vaginalis (TV-E650); originally cloned by Paces et al. (1992). This technique was applied to 426 women who visited Hanyang University Kuri Hospital, and its diagnostic value was compared with those of other detection methods. It was found that this PCR based method had a sensitivity and specificity of 100%, and that it could detect T. vaginalis in vaginal discharge fluid at a concentration as low as 1 cell per PCR mixture.
Detection by PCR was found to be specific for T. vaginalis, as no amplification was detected from the DNAs of other protozoans or Candida albicans (Table 3). These results indicate that PCR could be used as a specific and sensitive diagnostic tool for human trichomoniasis. In addition, PCR is believed to be useful for the diagnosis of trichomoniasis in men, because few trophozoites are generally detected in male reproductive organs (Kim and Kim, 1985).
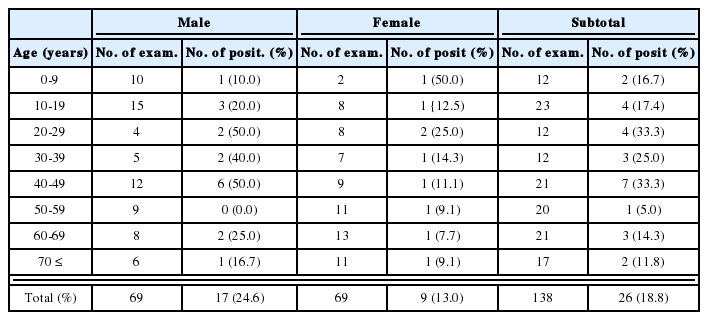
Comparison of trichomoniasis diagnostic methods in patients with suspected vaginitis (A group) and in women for screening by Papanicolaou smear (B group) (Ryu et al., 1999)
CHEMOTHERAPY
Metronidazole and other 5-nitroimidazoles are effective for treatment of human trichomoniasis and infections caused by other anaerobic protozoa and anaerobic bacteria. 5-Nitroimidazole derivatives such as metronidazole, tinidazole, ornidazole, and secnidazole have been evaluated for therapeutic effects on T. vaginalis infections. Choi (1976) compared the cure rate of a standard metronidazole course (250 mg administered orally 3 times daily for 7 days) with that of a single 2 gram dose of tinidazole. Their cure rates were 90% (metronidazole administration; 45 cases) and 97% (tinidazole; 45 cases), respectively. Thus, both drugs were found to be effective at treating trichomoniasis. Cho et al. (1976) and Ko et al. (1976) evaluated the efficacy of ornidazole (Tiberal@) for trichomonas vaginitis, and found that it produced excellent therapeutic results with a rapid clinical improvement and trophozoite disappearance. In addition, Soh et al. (1988) tested the effects of ornidazole on T. vaginalis morphology, and found that T. vaginalis propagation was retarded and that trophozoites bearing flagella were killed due to induced morphologic changes, i.e., polyribosome decomposition to a single ribosome, an increase in the cytoplasmic matrix, and the extrusion of protoplasm, which all might physiologically reduce protein synthesis and enzyme activity. You et al. (1985) assessed the effectiveness of a single 2 gram dose of seconidazole for T. vaginalis treatment, and obtained a cure rate of 96.8% 1 week after treatment, and main subjective symptoms disappearance within 2 or 3 days of treatment.
Metronidazole is both highly effective and approved by the WHO (1995b), yet it is listed as the second drug of choice by the Center for Disease Control in the USA because of its potential carcinogenicity in rats and mutagenicity in bacteria when administered at high doses for protracted periods of time (Legator et al., 1975; Rosenkranz and Speck, 1975). In addition, reports on metronidazole-resistant T. vaginalis have recently increased (Sobel et al., 1999). The authors of the present study previously found 3 Korean vaginitis cases caused by metronidazole-resistant T. vaginalis (data not shown). In addition, metronidazole sometimes causes adverse effects, e.g., myopia, neuralgia, and allergic dermatitis (Moldwin, 1992), and thus new anti-trichomonal drugs are probably required.
Metronidazole enters trichomonads by diffusion and is then activated by a one-electron reduction to its cytotoxic nitro-radical anion form in hydrogenosomes. Sodium nitrite has been extensively used in the curing and preservation of meats and in meat production. It functions as a color fixative and flavor developer, but it also acts as a preservative (Binkerd and Kolari, 1975). Sodium nitrite has been well documented to inhibit a wide variety of bacteria, including Clostridia spp. and Staphylococcus aureus (Buchanan and Solberg, 1972; O'Leary and Solberg, 1976). Moreover, the addition of sodium nitrite to a suspension of C. sporogenes resulted in the growth inhibition of clostridia and of the phosphoroclastic system, which consists of PFOR (pyruvate ferredoxin oxidoreductase), ferredoxin and hydrogenase (Payne et al., 1990). Hydrogenosomal pyruvate metabolism in trichomonads is strikingly similar to the bacterial system, as demonstrated by Clostridia sp. (Müller, 1980). Ryu and Lloyd (1995) and Ryu et al. (1995) nvestigated the action of sodium nitrite and of other nitrosyl complexes, i.e., sodium nitroprusside and Roussin's black salt, on the growth of metronidazole-sensitive and resistant strains of T. vaginalis. All 3 chemicals were found to inhibit the growth of T. vaginalis, i.e., sodium nitrite at 8 mM, sodium nitroprusside at 1.2 mM, and Roussin's black salt at 0.2 mM. Moreover, these agents were found to have similar cytotoxic effects on metronidazole-sensitive (KT9) and resistant (CDC85) isolates.
Iron is essentially required for life, because it is an essential component in many metabolic processes, including oxygen and electron transport, and DNA synthesis (Conrad et al., 1999). In cases of trichomoniasis infection, symptomatic aggravation after menstruation is probably related to iron availability and increased vaginal acidity (Ryu et al., 2001a). 2,2'-dipyridyl, a ferrous iron-chelator has been reported to suppress T. vaginalis growth, and to reduce adhesin synthesis and the adherence of T. vaginalis to epithelial cells (Ryu et al., 2001a). Ryu et al. (2001b) tested the in vitro activity of 2,2'-dipyridyl, an iron-chelator, against clinical isolates of T. vaginalis, C. albicans, and Gardnerella vaginalis and compared these findings with those of four other vaginal suppositories, i.e., ornidazole, clotrimazole, povidone-Iodine, and Cenacert® (methylbenzethonium chloride mixed with 9-aminoacrydine undecylenate and hydrochloric acid N-myristyl-3-hydroxy butyl amine). The 2,2'-dipyridyl was found to kill T. vaginalis and G. vaginalis at concentrations of 410 µg/ml and 205 µg/ml, respectively, but it was less active against C. albicans (80% inhibition at 410 µg/ml); moreover, the inhibitions of these 3 pathogens by 2,2'-dipyridyl were similar to their inhibitions by clotrimazole. These results suggest that 2,2'-dipyridyl has an inhibitory effect on C. albicans, G. vaginalis, and T. vaginalis, i.e., the 3 known causes of vulvovaginitis
Recently various herbal medications have been used to kill T. vaginalis in vitro. Eighteen aqueous and 2 ethanol extracts of traditional herbal medicines used to treat trichomoniasis in the Republic of Korea were assessed for their anti-trichomonas activities. Two extracts (Sophorae radix, Phellodendri cortex) showed evident anti-trichomonas activity at 8 mg/ml, and the ethylacetate fraction of Sophorae radix showed antitrichomonas activity at only 400 µg/ml. These findings indicate that Sophorae radix is potentially a potent safe antitrichomonas agent (Park et al., 2005). In addition, Kim et al. (2003), Choi et al. (2002), and Park et al. (2004) found that a kalopanaxsaponin A isolate from Kalopanax pictus, and extracts of Sophora flavescens, and Gleditsia sinensis have an anti-protozoal effect on T. vaginalis by inhibiting cell growth and impairing protein synthesis, respectively, and Ryang et al. (2001) found that an extract of Gentiana scabra var buergeri also inhibits the growth of T. vaginalis.
ACKNOWLEDGMENTS
We would like to thank laboratory people of Department of Parasitology, Hanyang University College of Medicine. This study was partially supported by the Hanyang University, the Republic of Korea, made in the program year of 2001.