Molecular detection of Toxoplasma gondii in ticks and their respective host dogs
Article information
Abstract
We identified the presence of Toxoplasma gondii in ticks and their host dogs, and assess the potential role of ticks as reservoirs for this pathogen. A total of 1,230 feeding ticks were collected from 340 dogs. The tick species identified included Haemaphysalis longicornis, H. flava, and Ixodes nipponensis. Detection of the T. gondii B1 gene occurred in 2 dogs (0.6%) and 4 tick pools (0.9%). Genotyping confirmed the presence of the I/III genotype. This study is the first to report the molecular detection of T. gondii in both canine ticks and their hosts. Our findings offer important insights into the dynamics of T. gondii transmission between vectors and their hosts.
The zoonotic parasite Toxoplasma gondii is known for its ability to infect a wide range of animals and humans globally, with an infection rate that may affect between 30% and 50% of the human population [1]. This parasite poses significant concerns in veterinary medicine due to its impact on both domestic and wild animals [2]. T. gondii can utilize all warm-blooded animals as intermediate hosts, while members of the Felidae family, including domestic cats, serve as definitive hosts [3].
In Korea, T. gondii has been detected in companion animals, such as cats and dogs [4,5]. Given that herbivorous animals are commonly affected by this parasite, it is improbable that oral transmission (e.g., through consumption of infected meat or exposure to cat feces) is the sole route of infection, suggesting the possibility of other transmission pathways. This has led to the hypothesis that blood-feeding arthropods like ticks could be involved in the transmission of T. gondii [6]. In Korea, T. gondii has recently been found in tick species such as Haemaphysalis longicornis, H. flava [7], and Ixodes turdus [8]. Other countries have also reported T. gondii in ticks like H. longicornis [9] and I. ricinus [10].
Ticks are ubiquitous and serve as primary vectors for a range of pathogens that cause severe diseases in both humans and animals [11]. T. gondii has been observed to persist in H. longicornis ticks for over 15 days, with transmission to hosts occurring through ingestion of infected ticks rather than blood feeding [9].
Despite the potential for tick-borne transmission of T. gondii, this aspect remains underexplored. As evidence of tick-borne diseases’ role in various health issues grows, it is increasingly important for clinicians to consider toxoplasmosis in the differential diagnosis of tick-borne diseases [6]. This study, therefore, aimed to assess the prevalence of T. gondii in dogs and the ticks feeding on them using molecular techniques.
Blood samples were collected from dogs by licensed veterinarians at local clinics and animal shelters during routine surveillance, treatment, monitoring, or regular medical check-ups, with verbal consent obtained from the respective owners. This study was beyond the purview of the Institutional Animal Care and Use Committee at Kyungpook National University, as Institutional Animal Care and Use Committee at Kyungpook National University evaluates laboratory animals maintained in indoor facilities and does not regulate research involving outdoor animals. In addition, removal of ticks from dogs is neither harmful nor against animal welfare.
A total of 340 blood samples were randomly selected from clinically normal dogs across various regions of Korea, collected during monitoring and surveillance activities from 2019 to 2021. The regions were classified as northern, central, and southern. In the northern region, samples were collected from Gyeonggi and Gangwon Provinces. Within the central region, collection was performed in Chungcheong Province. Furthermore, samples in the southern region were collected from Gyeongsang and Jeolla Provinces. The collected data included information on the dogs’ sex, age, source, region, and season, which were used in subsequent analyses.
A total of 1,230 ticks were gathered from 340 dogs, with each dog hosting between 3 and 8 ticks. Some dogs exhibited mild skin redness around the tick bite sites. Ticks were carefully removed from the head, neck, abdominal area, and mouthparts of the dogs using fine forceps and then stored in vials containing 70% ethanol. Initially, ticks were identified at the species level and classified morphologically according to their developmental stages [12]. The ticks were then pooled by dog, species, and developmental stage, forming 423 tick pools (ranging from 1 to 7 ticks per pool). The species identification was further confirmed through molecular characterization using PCR with specific primers targeting the mitochondrial cytochrome c oxidase subunit I (COI) gene [13].
Genomic DNA was extracted from the dog blood samples and the pooled ticks using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. PCR amplification was performed using an AccuPower HotStart PCR Premix kit (Bioneer, Daejeon, Korea). Nested PCR was conducted on both dogs and ticks to detect T. gondii by amplifying the B1 gene, as previously described [14]. A positive control using a T. gondii sample isolated from cattle in Korea [15] and a negative control without a DNA template were included in the assays.
Amplicons were purified using the QIAquick Gel Extraction Kit (Qiagen), ligated into pGEM-T Easy vectors (Promega, Madison, WI, USA), transformed into Escherichia coli DH5α competent cells (Thermo Fisher Scientific, Wilmington, DE, USA), and incubated overnight at 37°C. Plasmid DNA was extracted from the transformed cells using a plasmid miniprep kit (Qiagen) according to the manufacturer’s protocol.
Selected recombinant clones were sequenced by Macrogen (Seoul, Korea). The sequences were analyzed and aligned using CLUSTAL Omega v1.2.1. Alignment edits were made using BioEdit v7.2.5 software. Phylogenetic analysis was performed using MEGA v6.0, employing the maximum likelihood method with the Kimura 2-parameter distance model. The aligned sequences were analyzed using a similarity matrix, and the robustness of the phylogenetic trees was evaluated by bootstrap analysis with 1,000 replicates.
Statistical analyses were conducted using GraphPad Prism v5.04. Differences among the groups were evaluated using the chi-square test, with a P-value of <0.05 considered statistically significant. The 95% confidence interval (CI) was calculated for all estimates.
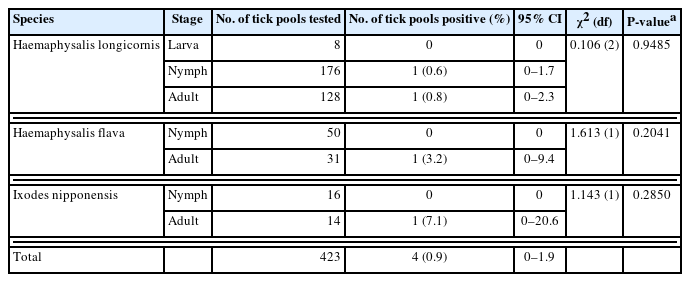
A total of 1,230 ticks, organized into 423 pools, were systematically collected from dogs. These ticks represented 2 genera and 3 species: H. longicornis, H. flava, and I. nipponensis. Universal primers targeting the COI gene were employed to amplify 710-bp fragments from the tick samples. The resulting amplicons were sequenced and carefully analyzed to correct potential errors in morphological identification, particularly in the case of nymphs and larvae. The COI gene sequences were classified into 3 distinct groups based on nucleotie identity, demonstrating close genetic relationships with H. longicornis (99.0%–100% nucleotide identity), H. flava (98.9%–100%), and I. nipponensis (98.8%–100%). A comprehensive phylogenetic tree was constructed using the COI gene sequences from various ticks archived in GenBank and the sequences obtained in this study. The ticks from this study grouped into 3 clades, corresponding to the 3 identified species (Fig. 1): H. longicornis (73.8%, 312/423 pools), H. flava (19.1%, 81/423 pools), and I. nipponensis (7.1%, 30/423 pools) (Table 1).
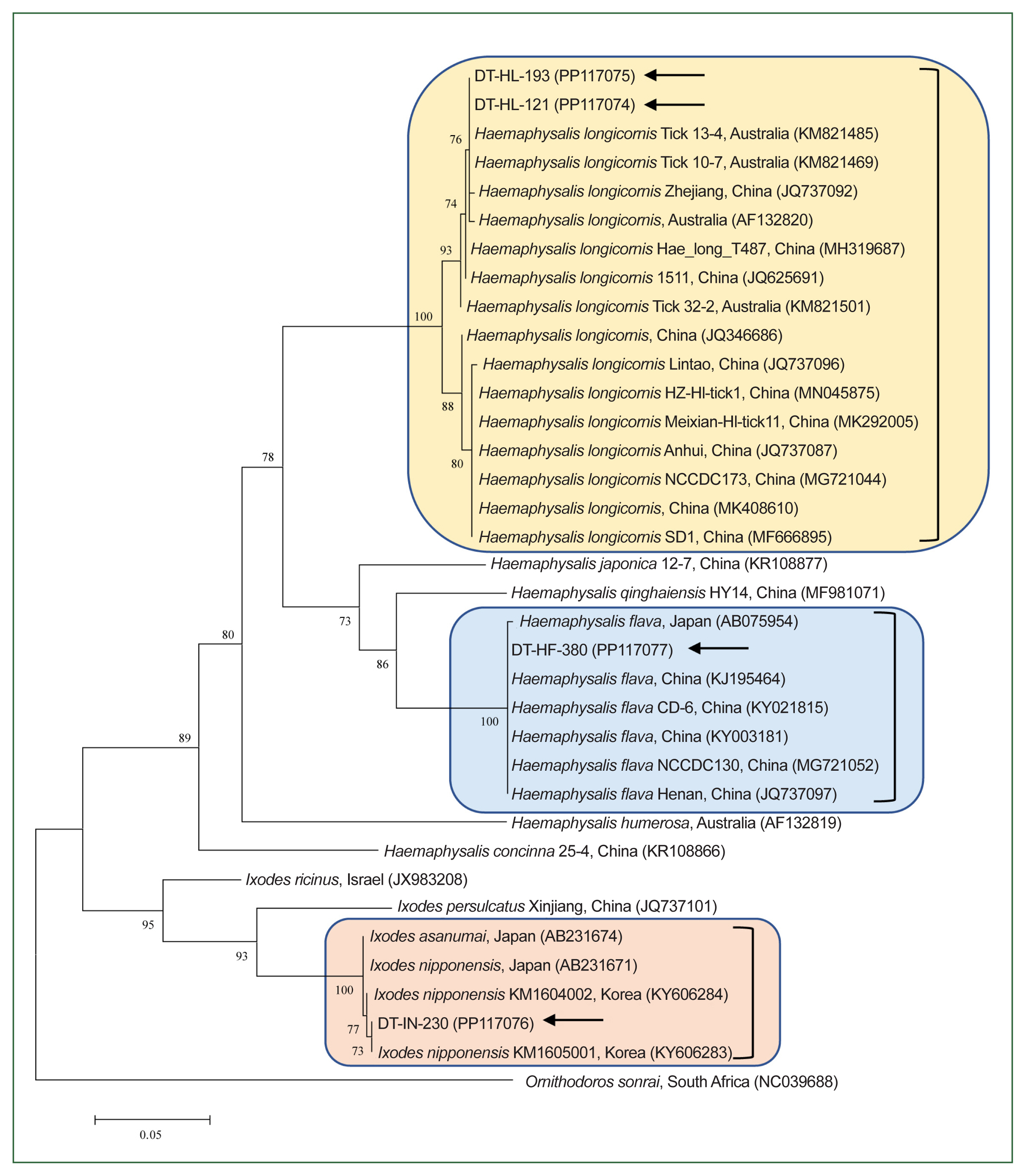
Maximum likelihood phylogenetic tree constructed using the mitochondrial cytochrome c oxidase subunit I gene sequences for the molecular identification of the ticks collected in our study. Ornithodoros sonrai was used as the outgroup. Black arrows indicate the sequences obtained and subsequently analyzed in our study. GenBank accession numbers for additional sequences are provided alongside their respective names. Numbers on the branches indicate the percentage bootstrap support (1,000 replicates). The scale bar represents the phylogenetic distance.
Among the samples analyzed, 0.9% (4/423, 95% CI=0–1.9) of the tick pools and 0.6% (2/340, 95% CI=0–1.4) of the dogs tested positive for the T. gondii B1 gene (Tables 1, 2, respectively). Each of the 4 tick pools that tested positive for T. gondii was collected from different dogs (1.2%, 4/340). Two of these tick pools were collected from 2 T. gondii-positive dogs, while the other 2 were collected from T. gondii-negative dogs. The COI PCR results indicated that the prevalence of T. gondii was 0.6% (1/176, 95% CI=0–1.7) in nymphs, 0.8% (1/128, 95% CI=0–2.3) in adult H. longicornis, 3.2% (1/31, 95% CI=0–9.4) in adult H. flava, and 7.1% (1/14, 95% CI=0–20.6) in adult I. nipponensis. Although no significant differences in prevalence were observed between different stages, the prevalence tended to be higher in adults compared to nymphs. Among the dogs, no significant differences in T. gondii prevalence were observed between males (0.6%, 1/165, 95% CI: 0–1.7) and females (0.6%, 1/175, 95% CI=0–1.8). In terms of age, T. gondii was most prevalent in dogs under 3 years old (1.6%, 1/62, 95% CI=0–4.7), followed by those aged 3–7 years (0.6%, 1/176, 95% CI=0–1.7), and was not detected in dogs over 7 years old. Although the differences were not statistically significant, younger dogs tended to have higher prevalence rates than older dogs. T. gondii prevalence was significantly higher in shelter dogs (1.8%, 2/110, 95% CI=0–4.3; P=0.0406) compared to companion dogs (0%, 0/230). The detection of T. gondii was significantly higher in the southern region (2.4%, 2/84, 95% CI=0–5.6; P=0.0466) and during the spring season (3.1%, 2/65, 95% CI=0–7.3; P=0.0365), indicating potential environmental or ecological factors affecting its prevalence.
The 2 H. longicornis COI gene sequences from this study showed 98.0%–100% nucleotide identity with known H. longicornis COI sequences and placed them within the H. longicornis clade on neighboring branches (Fig. 1). Similarly, the H. flava and I. nipponensis COI gene sequences demonstrated 99.1%–100% and 98.9%–99.7% identity, respectively, with known sequences of the same species (Fig. 1).
Phylogenetic analysis of the 6 T. gondii B1 gene sequences from this study (99.8%–100% identity) revealed that they clustered with known T. gondii sequences (99.1%–100% identity; Fig. 2). The detected T. gondii strains were identified as genotype I/III. All representative sequences were submitted to GenBank (accession No. PP117076 (I. nipponensis), PP117077 (H. flava), PP117074 and PP117075 (H. longicornis), and PP096887–PP096891 and PR133183 (T. gondii)).
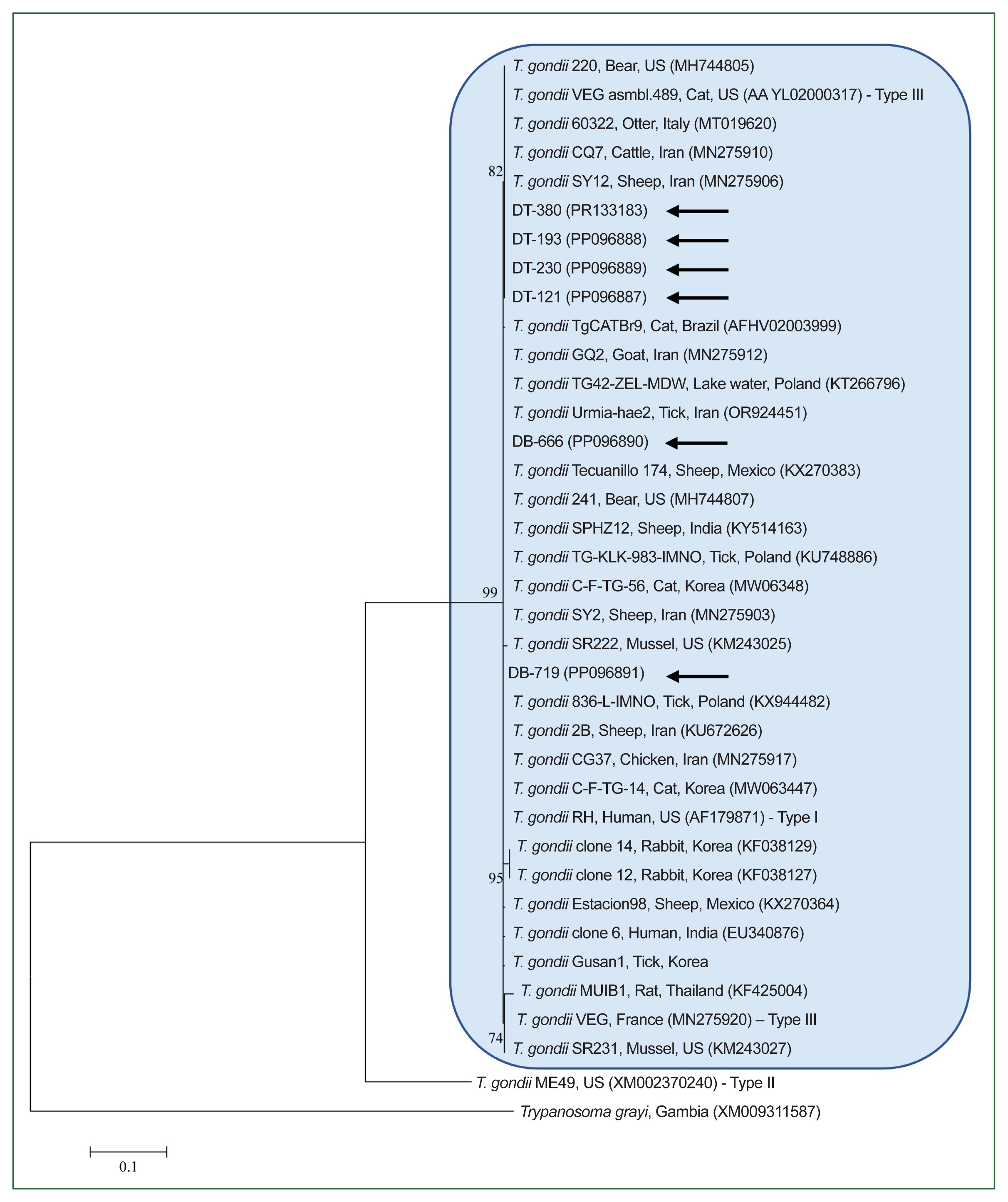
Maximum likelihood phylogenetic tree of Toxoplasma gondii constructed based on B1 gene sequences. Black arrows indicate the sequences analyzed in this study. Trypanosoma grayi was used as the outgroup. GenBank accession numbers for additional sequences are provided alongside their respective names. Numbers on the branches indicate the percentage bootstrap support (1,000 replicates). The scale bar represents the phylogenetic distance.
We identified 3 tick species on Korean dogs: H. longicornis (73.8%), H. flava (19.1%), and I. nipponensis (7.1%). These findings are consistent with a previous study conducted in this country, which reported that the majority of ticks found on dogs belonged to the genus Haemaphysalis (97.8%), including H. longicornis (48.9%) and H. flava (17.3%). Other species identified in that study included I. nipponensis (1.7%) and Rhipicephalus sanguineus (0.5%) [16]. H. longicornis is typically associated with herbaceous and grassy environments, H. flava with forested areas, and I. nipponensis has been found in both habitats across Korea [17].
Toxoplasma gondii infection is primarily acquired through the consumption of raw meat containing T. gondii tissue cysts or by ingesting food or water contaminated with oocysts shed by definitive host cats [18]. In Korea, a study used PCR to determine T. gondii prevalence in feral cats (47.2%, 50/106) and in German shepherd dogs used for guarding or hunting in rural areas (46.4%, 64/138) [4]. Another study conducted from 2017 to 2019 reported the prevalence of T. gondii antibodies in dogs (1.6%, 54/3,359) and cats (5.9%, 78/1,312) using ELISA, and the prevalence of T. gondii DNA in dogs (0.3%, 8/2,660) and cats (0.2%, 10/4,432) using PCR [5]. In our study, T. gondii was detected in 2 dogs (0.6%) by PCR, a finding that aligns with the low detection rate reported in dogs by Park et al. [5]. We also found that T. gondii was only detected in shelter dogs (1.8%), but not in companion dogs, with the difference being statistically significant. This observation parallels findings that stray cats (14.1%) and stray dogs (5.6%) exhibit higher antibody prevalence rates for T. gondii compared to domestic cats (2.3%) and domestic dogs (0.04%) in Korea [5]. Stray animals likely have more opportunities for T. gondii exposure in the outdoor environment than pets. Unfortunately, follow-up studies on the 2 shelter dogs that tested positive were not possible, as both were euthanized after blood sampling. Regionally, T. gondii was detected only in the southern part of Korea and only during the spring season, suggesting that both location and season are significant factors influencing T. gondii prevalence in dogs in this region. The warmer and more humid climate of southern Korea may enhance the survival of T. gondii oocysts in the environment, thereby increasing transmission opportunities to intermediate hosts, including dogs. Seasonal differences in prevalence, with higher rates observed in spring, may reflect increased exposure to contaminated environments during this period. This finding has epidemiological significance, as it implies that certain regions and seasons pose a higher risk of T. gondii transmission in Korea. Given the significant regional and seasonal variation observed, this study highlights the importance of monitoring T. gondii prevalence in different environments in Korea. Targeted control efforts in high-risk regions, such as the southern areas during spring, could be beneficial for reducing T. gondii transmission in both animals and humans. Further longitudinal studies are warranted to investigate these temporal and spatial patterns more comprehensively and to develop appropriate prevention strategies.
Toxoplasma gondii is a well-known pathogen causing toxoplasmosis in both animals and humans. Although ticks have been identified as potential carriers of T. gondii, their role in the transmission of this parasite has been largely underestimated, potentially due to a lack of awareness and available information [6]. The detection of T. gondii DNA in unfed adult ticks suggests that vertical transmission through transstadial or transovarial routes might occur, contributing to the relatively common occurrence of toxoplasmosis in small mammals (such as rodents), birds, and large herbivores. Supporting evidence for this possibility has been reported in previous studies. For instance, Zhou et al [9]. demonstrated that H. longicornis ticks could harbor T. gondii DNA for at least 15 days, suggesting the potential for vertical transmission pathways such as transstadial survival. Similarly, a previous study identified T. gondii DNA in unfed larvae and nymphs of I. ricinus ticks, reinforcing the hypothesis of transstadial and possibly transovarial transmission [19]. Furthermore, Ben-Harari highlighted the broader role of ticks as vectors for T. gondii and suggested that these vertical transmission pathways may explain the parasite’s persistence in various host species [6]. These animals are unlikely to acquire infection orally through meat or cat feces but could become infected through bites from infected ticks [6].
In Korea, T. gondii was detected in 4.1% (13/314) of ticks collected from vegetation (H. longicornis, n=11; H. flava, n=2) in 2014–2016 [7] and in 0.4% (1/237 tick pools) of I. turdus ticks collected from migratory birds in 2010–2011 and 2016 [8]. Studies from other countries have also reported T. gondii in various tick species, such as H. longicornis in China (9.2%, 39/422) [9], and I. ricinus in Poland collected from ponies (3%, 52/1,737) and vegetation (10.2%, 38/371) [10].
In our study, most ticks were collected during the nymph stage, and T. gondii was detected in 4 engorged tick pools (0.9%) collected from 4 individual host dogs. The prevalence was higher in adult tick (1.7%) than in nymph tick (0.4%), with no T. gondii detected in larvae. These results align with previous studies that reported higher T. gondii prevalence in adult ticks compared to other stages, suggesting that adult ticks are more susceptible to T. gondii infection [7,9]. Furthermore, the varying prevalence of T. gondii at different developmental stages may be associated with the frequency of blood-feeding ticks [9].
Of the 4 tick pools infected with T. gondii, 2 pools (6 H. longicornis nymphs and 3 I. nipponensis adults) were from dogs that also tested positive for the parasite, while the other 2 pools (4 H. longicornis adults and 3 H. flava adults) were from T. gondii-negative dogs. Phylogenetic analysis revealed 99.8% similarity between the T. gondii sequences from the tick pools and the corresponding dog samples. However, the transmission route in these cases could not be confirmed, as no evidence of transmission via ingestion or blood feeding was observed. It is hypothesized that T. gondii detection in ticks may indicate prior feeding on other intermediate hosts, such as rodents or birds, from which the ticks acquired tachyzoites through blood meals [10]. Although the available studies are limited, they suggest that ticks could play a role in the transmission cycle of T. gondii, either directly through blood feeding or indirectly through host ingestion of an infected tick [6].
To the best of our knowledge, this is the first study to report the detection of T. gondii in both tick vectors and their respective hosts, and the first global identification of T. gondii in I. nipponensis. While no experimental evidence of T. gondii transmission between ticks and their hosts was observed, our findings indicate the potential role of ticks as vectors in the transmission of T. gondii. Further research is necessary to understand how T. gondii survives in natural environments and to explore the potential transmission of T. gondii by ticks to other hosts through blood feeding or ingestion.
Toxoplasma strains are classified into 3 genotypes based on their virulence levels: Type I (highly virulent), Type II (less virulent), and Type III (avirulent) [20]. Our genotyping results belonged to genotype I/III, clustering within the same phylogenetic group as the highly pathogenic Type I and moderately pathogenic Type III strains. Further phylogenetic analyses are warranted to distinguish Type I more definitively from Type III.
Our study uncovered a relatively low prevalence of T. gondii in both ticks and their host dogs. This investigation is the first to employ molecular methods to compare the presence of T. gondii in canine ticks and their hosts. Although many aspects of the epidemiology of T. gondii infection in ticks remain unclear, and studies on the potential transmission of T. gondii from infected ticks to mammalian hosts are limited, our findings suggest that ticks could be involved in the dissemination of this parasite. Given the significant impact of T. gondii on both human and animal health, it is crucial to determine whether ticks serve as a vector for this parasite. The findings from our study contribute valuable information to the understanding of T. gondii transmission dynamics between vectors and hosts. Additionally, we propose the possibility of a novel vector-borne transmission route for T. gondii in tick hosts.
Notes
Author contributions
Conceptualization: Kwak D
Formal analysis: Seo MG
Validation: Seo MG
Writing – original draft: Seo MG
Writing – review & editing: Kwak D
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (Grant No. NRF-2016R1D1A1B02015366).