Clonorchis sinensis dopamine transporter (CsDAT) facilitates dopamine uptake
Article information
Abstract
Clonorchis sinensis is a liver fluke that causes clonorchiasis, a significant public health concern in East Asia, closely associated with hepatobiliary diseases. Dopamine is an essential neurotransmitter involved in neuromuscular signaling, and its uptake by trematodes may contribute to parasite physiology and survival. This study aimed to characterize the dopamine transporter CsDAT in C. sinensis by synthesizing cDNA from adult worms and expressing it in Xenopus laevis oocytes; subsequently, uptake assays were conducted using radiolabeled dopamine. Functional assays confirmed that CsDAT mediates dopamine uptake in a sodium-dependent manner. The uptake was saturable and exhibited Michaelis-Menten kinetics with a Michaelis constant of 454.5 nM and a maximum uptake rate of 1,422.5 fmol/oocyte/h. CsDAT efficiently transported dopamine with high affinity, indicating its physiological relevance in the parasite. A 3-dimensional model of CsDAT was constructed to examine its structural features. The predicted structure contained a conserved substrate-binding pocket similar to that of other known neurotransmitter transporters. Molecular docking simulations showed that dopamine stably fits within the binding pocket. The key amino acid residues formed hydrogen bonds and hydrophobic interactions with dopamine. Interestingly, dopamine and several inhibitors demonstrated higher binding affinity to CsDAT than the human dopamine transporter. This study provides the first functional and structural insights into CsDAT. The higher inhibitor-binding affinity of CsDAT compared to human dopamine transporter suggests its potential for use in therapeutic exploration. Targeting CsDAT may facilitate the development of new therapeutic agents against clonorchiasis with minimal off-target effects on the human nervous system.
Introduction
Clonorchis sinensis is a parasitic liver fluke that causes clonorchiasis, an endemic disease in several East Asian countries, particularly China, Korea, and Vietnam [1]. It is estimated that 15–20 million people are currently infected with clonorchiasis, making it a significant public health concern [1]. Humans are infected with this disease by consuming raw or undercooked freshwater fish that carry the infective form of the parasite, known as the metacercariae. The metacercariae excyst in the duodenum and rapidly migrate into the intrahepatic bile ducts after ingestion, where they mature into adult worms and survive for years. Chronic infection may lead to bile duct inflammation, gallstones, liver fibrosis, and in severe cases, cholangiocarcinoma [1].
Although the clinical effects of C. sinensis infection are well documented, the biology of the parasite remains poorly understood. The fluke contains dopamine, noradrenaline, acetylcholinesterase, and an acetylcholine-like substance, which are localized in its nervous system [2]. Despite its relatively simple structure, the nervous system plays an essential role in flatworms [3]. It helps control movement, sensory responses, feeding, and internal regulation [4]. However, the nervous system of the fluke has received less attention compared to other aspects of its life cycle. Understanding the functioning of the nervous system in parasitic flukes is crucial as it can reveal how parasites interact with their environment and sustain survival within the host.
Neurotransmitters are key components of the nervous system that mediate communication between nerve cells and other tissues. Several types of neurotransmitters have been identified in parasitic flatworms, including serotonin, acetylcholine, gamma-aminobutyric acid, and dopamine [5,6]. Notably, dopamine has been detected throughout the entire body and plays a crucial role in regulating muscle movement [7]. It generally acts as an inhibitor of motor activity, helping the parasite maintain precise control over its muscular system [8]. Research on Schistosoma mansoni, a blood fluke, has shown that dopamine can modulate both circular and longitudinal muscles, directly affecting the movement of the worm [9]. A similar role has been proposed in Fasciola hepatica, where dopamine-related genes and receptors have been identified [10].
Dopamine plays a wide range of roles in humans, including the regulation of motor control, motivation, mood, and hormone release [11]. It is synthesized from the amino acid tyrosine [12]. The key steps in its synthesis involve the conversion of tyrosine to L-dihydroxyphenylalanine by tyrosine hydroxylase, followed by decarboxylation into dopamine by aromatic L-amino acid decarboxylase [13]. Once released into the synaptic space, dopamine is cleared through reuptake by a specialized protein called the dopamine transporter (DAT) [14]. This transporter removes dopamine from the extracellular environment and returns it to the presynaptic neuron [14], thus helping to regulate dopamine levels and ensure proper signaling.
Genomic analysis of C. sinensis revealed the presence of key enzymes involved in dopamine biosynthesis, including aromatic L-amino acid decarboxylase, suggesting that the parasite can produce dopamine endogenously [15,16]. However, the overall dopaminergic signaling framework in this species remains largely uncharacterized. While the DAT, a major component of this system, has been studied in other parasitic flatworms such as S. mansoni, functional evidence in C. sinensis is lacking [17]. Thus, the identification and functional characterization of the transporters are essential for improving our understanding of the neural regulation in C. sinensis, particularly the dopaminergic pathway. This study elucidates the role of DAT in C. sinensis (CsDAT) through functional characterization, thereby enhancing our understanding of how parasites regulate dopamine signaling by confirming that CsDAT facilitates dopamine uptake. The findings of this study may serve as a basis for future research on how disrupting the DAT could affect C. sinensis without impacting the host.
Materials and Methods
Ethics statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of Inha University (approval No. INHA161208-460), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The mice were housed at the Inha University Animal Facility in accordance with the National Animal Care Policies (Accredited Unit, Ministry of Food and Drug Safety of Korea; unit number 36).
Chemicals
Radiolabeled compounds, including [3H] dopamine (33.7 Ci/mmol), [3H] deoxy-D-glucose (32.5 Ci/mmol), [3H] arginine (50.5 Ci/mmol), [3H] estrone sulfate (45 Ci/mmol), [14C] α-ketoglutaric acid (54.8 mCi/mmol), [14C] p-aminohippurate (52.7 mCi/mmol), [3H] taurocholic acid (15.5 Ci/mmol), and [14C] tetraethylammonium (3.5 mCi/mmol), were purchased from PerkinElmer Life Science (Boston, MA, USA). All experiments were conducted using commercially available analytical-grade chemicals and reagents obtained from commercial sources.
Sequence retrieval and computational analysis
The nucleotide sequence of the putative sodium-dependent CsDAT (accession No. GAA29481) was retrieved from the National Center for Biotechnology Information database. The deduced amino acid sequence corresponded to solute carrier family 6 member 3 (high-affinity DAT, SLC6A3). Homologous DAT genes from other fluke species were also obtained from the National Center for Biotechnology Information and UniProt. Multiple sequence alignments and phylogenetic tree construction were conducted using MEGA11 with the maximum-likelihood method and 1,000 bootstrap replications to enhance robustness.
The membrane topology was predicted using the TOPCONS server (https://topcons.cbr.su.se). Tertiary structure modeling of CsDAT was performed using AlphaFold 2 (https://alphafold.ebi.ac.uk/), and the resulting models were evaluated using Ramachandran plots and ERRAT to assess stereochemical quality and overall reliability. Galaxy Refine was used for structural refinement (https://galaxy.seoklab.org/). The final predicted structure was visualized using the PyMol package. The human DAT (hDAT) was retrieved from the Protein Data Bank (PDB ID: 8Y2D) and used in molecular docking simulations with CsDAT via AutoDock Vina to estimate binding affinities within the binding pocket. The substrates included DAT inhibitors, such as amfonelic acid, benztropine, bupropion, and vanoxerine, whose tertiary structures were obtained from the PubChem database. Molecular interaction characteristics were analyzed using the PLIP server (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index) and visualized with PyMol.
Preparation of adult C. sinensis worms
Adult C. sinensis worms were obtained through experimental infection. Pseudorasbora parva and Gnathopogon coreanus were collected from the Nam River in Jinju, Korea. The fish tissues were minced and digested with a solution containing 0.6% pepsin (750 U/mg) and 0.7% HCl (pH 2.0) at 37°C for 6 h under agitation. The digested material was filtered through a gauze and washed repeatedly with saline to recover metacercariae, which were then isolated under a microscope using a 200-μm stainless wire mesh.
Approximately 1,500 metacercariae were collected, and each of the 10 male FVB/NJ mice (5–6 weeks old; Central Lab Animal Inc., Seoul, Korea) was infected with 100 metacercariae. Six weeks after infection, the presence of eggs in feces was confirmed using the formalin-ether sedimentation method. Adult worms were harvested from the liver margins and bile ducts at 8 weeks after infection.
Cloning of the CsDAT gene
Total RNA (1 μg) extracted from adult worms was used for cDNA synthesis using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega, Madison, WI, USA), 10 mM dNTPs, and 2.5 μM oligo-dT primers in a 30 μl reaction. The reaction proceeded for 30 min at 42°C, followed by enzyme inactivation at 99°C for 5 min.
The full-length CsDAT gene was amplified using overlap extension PCR. Gene-specific primers were used to generate 4 overlapping segments (Supplementary Table S1). Each PCR reaction mixture consisted of 3 μl of dNTPs (2.5 mM each), 1 unit of Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA, USA), 6 μl of 5×PCR buffer, 1 μl each of forward and reverse primers (10 μM), and DNase/RNase-free water to a final volume of 30 μl under the following cycling conditions: 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 45 sec, and a final extension at 72°C for 5 min (Supplementary Fig. S1A).
The segments were gel-purified using a Gel Extraction Kit (GeneAll Biotechnology, Seoul, Korea), subcloned into the pTOP TA V2 vector (Enzynomics, Daejeon, Korea), and verified by sequencing (Macrogen, Seoul, Korea).
For final gene assembly, EcoRI-digested PCR fragments were used as templates for overlap extension PCR, which was performed using LA Taq DNA polymerase (Takara, Seoul, Korea). The reaction mixture for overlap extension PCR contained 1 μl of each PCR fragment, 3 μl of dNTPs, 2 units of LA Taq DNA polymerase, 3 μl of 10×PCR buffer, 2 μl of 25 mM MgCl2, and 1 μl each of the first forward and 4th reverse primers (10 μM each) to a final volume of 30 μl. The thermal cycling conditions were as follows: initial denaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 2 min, followed by a final extension at 72°C for 5 min (Supplementary Fig. S1B). The PCR product was subcloned into a TA cloning vector, and its sequence was confirmed using an ABI Prism 3730 sequencer (Macrogen).
cRNA synthesis and oocyte injection
The Xenopus laevis oocyte expression system, a well-established platform for studying transporter function, was used to assess the dopamine uptake properties [18]. The CsDAT functional expression was inferred from dopamine uptake activity, which consistently indicated the presence of the transporter at the membrane. The plasmid DNA containing CsDAT cDNA was fully digested with Xho I (Enzynomics) to generate a linear template for cRNA synthesis. In vitro transcription was performed using the mMESSAGE mMACHINE T7 kit (Thermo Fisher Scientific, Carlsbad, CA, USA). Approximately 50 ng of synthesized cRNA (50 nl) was microinjected into defolliculated X. laevis oocytes.
Egg-bearing X. laevis frogs were handled in accordance with the regulations of the Inha University Committee on Animal Resources (approval No. INHA250701-977). The frogs were housed in a glass water tank (650 mm×400 mm×550 mm) filled with dechlorinated tap water maintained at 18°C±2°C and fed twice a week. The frogs were anesthetized by immersion in ice water before oocyte harvesting. A small abdominal incision was made to remove the ovarian lobes. The ovarian tissues were manually divided into small clumps and incubated with gentle agitation in collagenase A (0.2%) dissolved in OR II solution (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES; pH 7.5) at room temperature for 1–2 h, until the epithelial, thecal, and follicular layers were dissolved.
Defolliculated stage V and VI oocytes were selected and injected with cRNA under a dissecting microscope, while control oocytes were injected with distilled water. All oocytes were incubated at 18°C in Barth’s solution (88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.4 mM CaCl2, 0.8 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES, pH 7.4), supplemented with 50 μg/mL gentamicin and 2 mM sodium pyruvate.
Dopamine uptake and efflux assay
Two to three days after cRNA injection, dopamine uptake was assessed using [3H] dopamine in ND96 solution (96 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.4) at 22°C–25°C for 1 h. The uptake reaction was terminated by washing the oocytes with ice-cold ND96; they were then washed 4 times to remove residual radiolabeled substrate. Each oocyte was dissolved in 10% sodium dodecyl sulfate and mixed with Ultima Gold AB scintillation cocktail (PerkinElmer). The radioactivity was measured with a MicroBeta 2 β-counter (PerkinElmer). The oocytes were preloaded with 30 nM [3H] dopamine for 90 min and then transferred to ND96 medium containing unlabeled dopamine (1 or 10 μM) or control medium to evaluate the trans-stimulation of efflux. Radioactivity was measured in the medium and oocytes after 60 min.
Kinetic analysis and statistical analysis
The kinetic parameters of CsDAT-mediated uptake were determined using the Michaelis-Menten equation: v=Vmax×[S]/(Km+[S]), where v is the uptake rate (fmol/h/oocyte), [S] is the substrate concentration (μM), Km is the Michaelis constant, and Vmax is the maximum uptake rate. Net transport rates were calculated by subtracting values obtained from non-infected control oocytes. The MULTI program was used to perform curve fitting, applying an iterative nonlinear least-squares method with the Damping Gauss-Newton algorithm. Lineweaver-Burk plots were generated by transforming the data into the 1/[S] vs. 1/v format for validation.
All statistical analyses were performed using Student t-test in GraphPad 8. A P-value of less than 0.05 was considered statistically significant (*), while values of 0.01 (**) and 0.001 (***) were considered highly and very highly significant, respectively.
Results
Structural features of the CsDAT
The open reading frame of the CsDAT cDNA (GAA29481) comprised 1,962 base pairs, encoding a 653-amino-acid protein with a predicted molecular mass of 73.3 kDa. Topology analysis of the deduced amino acid sequence revealed 12 putative transmembrane domains, characteristic of neurotransmitter transporters (Fig. 1A). The N-terminus (1–42 aa.) and C-terminus (589–653 aa.) flanked the transmembrane domain in the intracellular region. In addition, 5 potential N-glycosylation sites were identified at residues 174, 181, 189, 545, and 551, suggesting possible post-translational modifications that could affect protein folding, trafficking, or function.
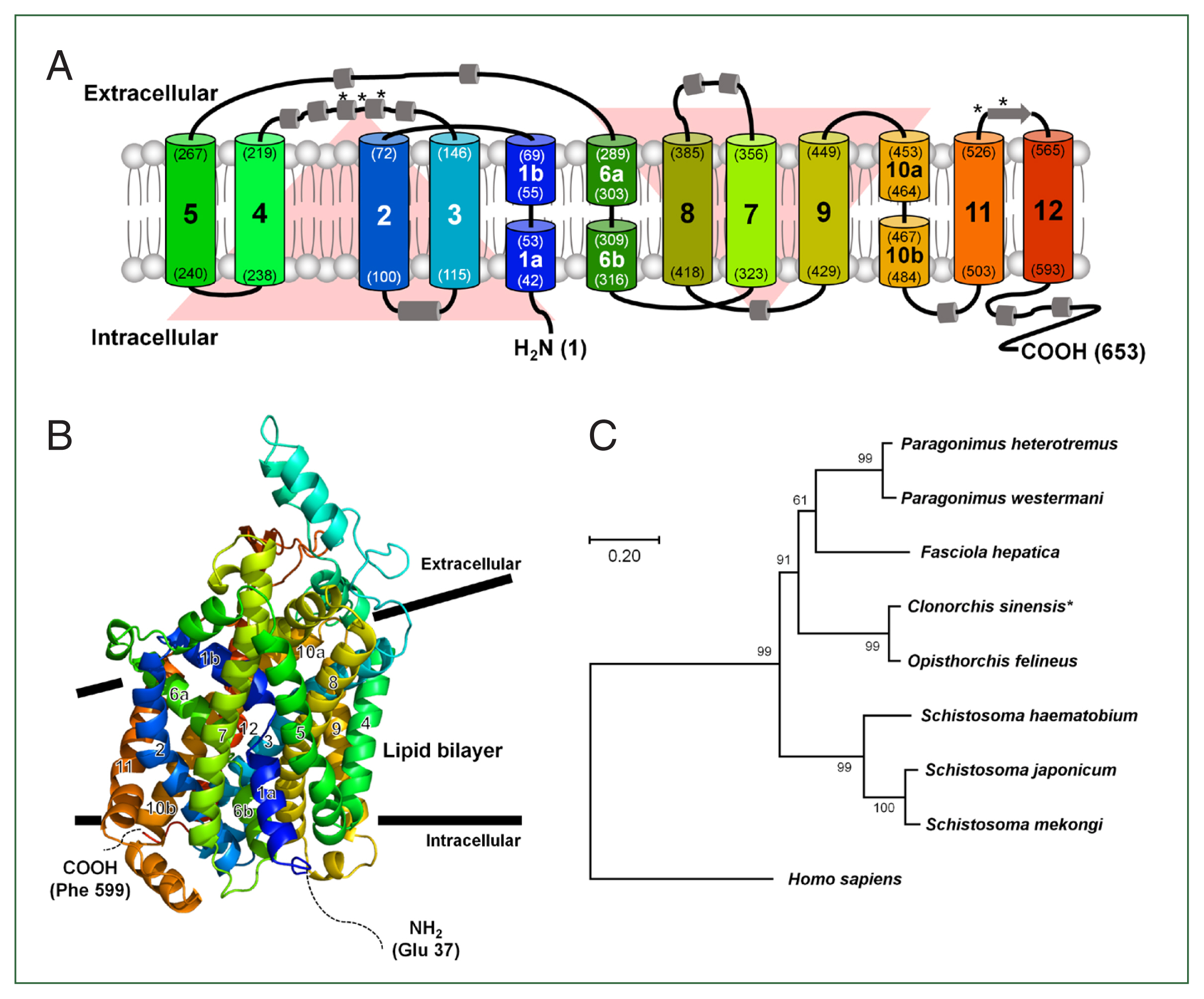
Predicted membrane topology and structural features of CsDAT. (A) Predicted membrane topology of CsDAT showing a 653-amino acid protein with 12 transmembrane helices (TM1–TM12) typical of neurotransmitter sodium symporter family transporters. Both the N- and C-termini are located in intracellular regions, and 5 potential N-glycosylation sites are identified at residues N174, N181, N189, N545, and N551. The helices are color-coded and numbered. The residue number indicates the boundaries of each helix. *, N-glycosylation site. (B) Predicted 3-dimensional structure of the CsDAT model. Transmembrane helices are color-coded and numbered. TM1, TM3, TM6, and TM8 form a central cavity that is likely involved in substrate and Na+ ion binding. The inward-facing broken helices in TM1 (1a and 1b) and TM6 (6a and 6b) may function as gates during the substrate translocation cycle. (C) Phylogenetic tree of CsDAT and related dopamine transporters from selected trematodes and mammals. CsDAT is clustered closely with the Opisthorchis felineus homolog, supporting its evolutionary conservation among parasitic flukes. The tree was constructed using the maximum-likelihood method with bootstrap values indicated at each node. Dopamine transporter sequences were obtained from the following species: Clonorchis sinensis (GAA29481), Opisthorchis felineus (TGZ63160), Paragonimus heterotremus (KAF5405469), Paragonimus westermani (KAF8563108), Fasciola hepatica (THD23603), Schistosoma haematobium (XP_035585913), Schistosoma japonicum (TNN07223), Schistosoma mekongi (KAK4475557), and Homo sapiens (AAC41720).
Further structural analysis revealed that the transmembrane helices TM1, TM3, TM6, and TM8 were arranged to form a central binding cavity, consistent with the substrate-binding architecture observed in other DAT homologs. This cavity was presumed to accommodate dopamine and co-transported Na+ ions, reflecting the conservation of the ion-dependent substrate transport mechanism. Notably, TM1 (1a and 1b) and TM6 (6a and 6b) exhibited inward-facing discontinuities characteristic of broken helix motifs, which are known to play a critical role in the alternating access transport cycle gating transitions in mammalian DATs. These structural features, as illustrated in the predicted 3-dimensional model (Fig. 1B), suggest that CsDAT mediates dopamine uptake via a similar conformational mechanism.
A phylogenetic analysis was conducted using transporter sequences from other trematodes to explore the evolutionary relationship of CsDAT. The result showed that CsDAT clustered closely with DAT homologs from Opisthorchis felineus, indicating a conserved evolutionary lineage among parasitic flukes (Fig. 1C). Sequence identity analysis showed that CsDAT shared 60.3%–93.7% amino acid similarity with DATs from other flukes and approximately 40.9% identity with mammalian counterparts (Supplementary Table S2), indicating functional conservation despite evolutionary divergence.
Substrate selectivity of the CsDAT
To evaluate the substrate specificity of CsDAT, [3H] dopamine uptake was measured in X. laevis oocytes with CsDAT expression. Dopamine uptake in CsDAT-expressing oocytes (mean±SE, 41.9±2.9 fmol/oocyte/h) was significantly higher than that in control oocytes (6.1±1.3 fmol/oocyte/h), corresponding to an approximately 7-fold increase (Fig. 2). In contrast, CsDAT did not mediate the uptake of other tested solutes, including [3H] deoxy-D-glucose, [3H] arginine, [3H] estrone sulfate, [3H] taurocholic acid, [14C] α-ketoglutaric acid, [14C] p-aminohippurate, and [14C] tetraethylammonium (Fig. 2). These results indicate that CsDAT exhibits high substrate selectivity for dopamine and does not transport structurally unrelated amino acids, dicarboxylate, steroid hormones, organic anions, or bile salts.
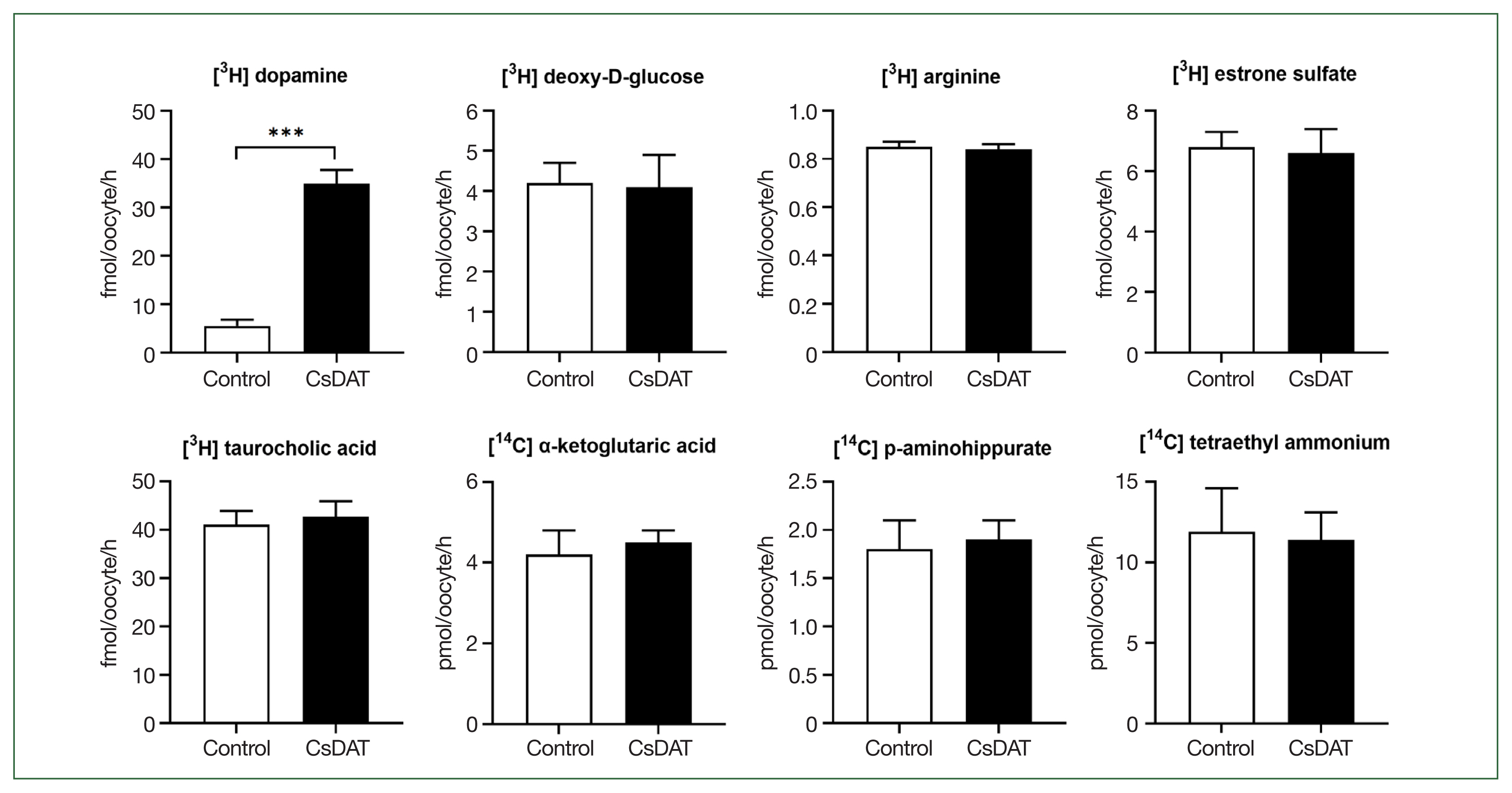
CsDAT-mediated uptake of dopamine. The uptake rates of the radiolabeled compounds were measured in water-injected (control, white bar) and CsDAT-expressing (black bar) oocytes for 1 h (mean±SE, n=8–10). The concentrations of substrates used were as follows: [3H] dopamine, 30 nM; [3H] deoxy-D-glucose, 150 nM; [3H] arginine, 100 nM; [3H] estrone sulfate, 50 nM; [3H] taurocholate, 200 nM; [14C] α-ketoglutaric acid, 5 μM; [14C] p-aminohippurate, 10 μM; and [14C] tetraethyl ammonium, 10 μM. Significant differences were observed using the Student t-test. ***P<0.001.
Characterization of CsDAT-mediated transport of dopamine
We examined [3H] dopamine uptake in X. laevis oocytes under various conditions to characterize the transport properties of CsDAT. The uptake increased with longer expression times following injection (24–72 h) (Fig. 3A) and with extended incubation durations (15–75 min) (Fig. 3B), indicating that CsDAT actively mediates dopamine translocation across the membrane. The oocytes were preloaded with [3H] dopamine and incubated in a buffer containing excess unlabeled dopamine (30-fold and 300-fold) to assess whether CsDAT also mediates dopamine efflux. No increase in radioactivity was observed, indicating that CsDAT does not facilitate dopamine efflux under these conditions (Fig. 3C). The uptake of [3H] dopamine by CsDAT-expressing oocytes exhibited saturable kinetics, consistent with the Michaelis-Menten model (Fig. 3D). Nonlinear regression analysis revealed a Km of 454.5 nM and a Vmax of 1,422.5 fmol/oocyte/h, indicating high-affinity and efficient transport. These kinetic parameters were further supported by the transformation of the Eadie-Hofstee plot (Fig. 3D, inset).
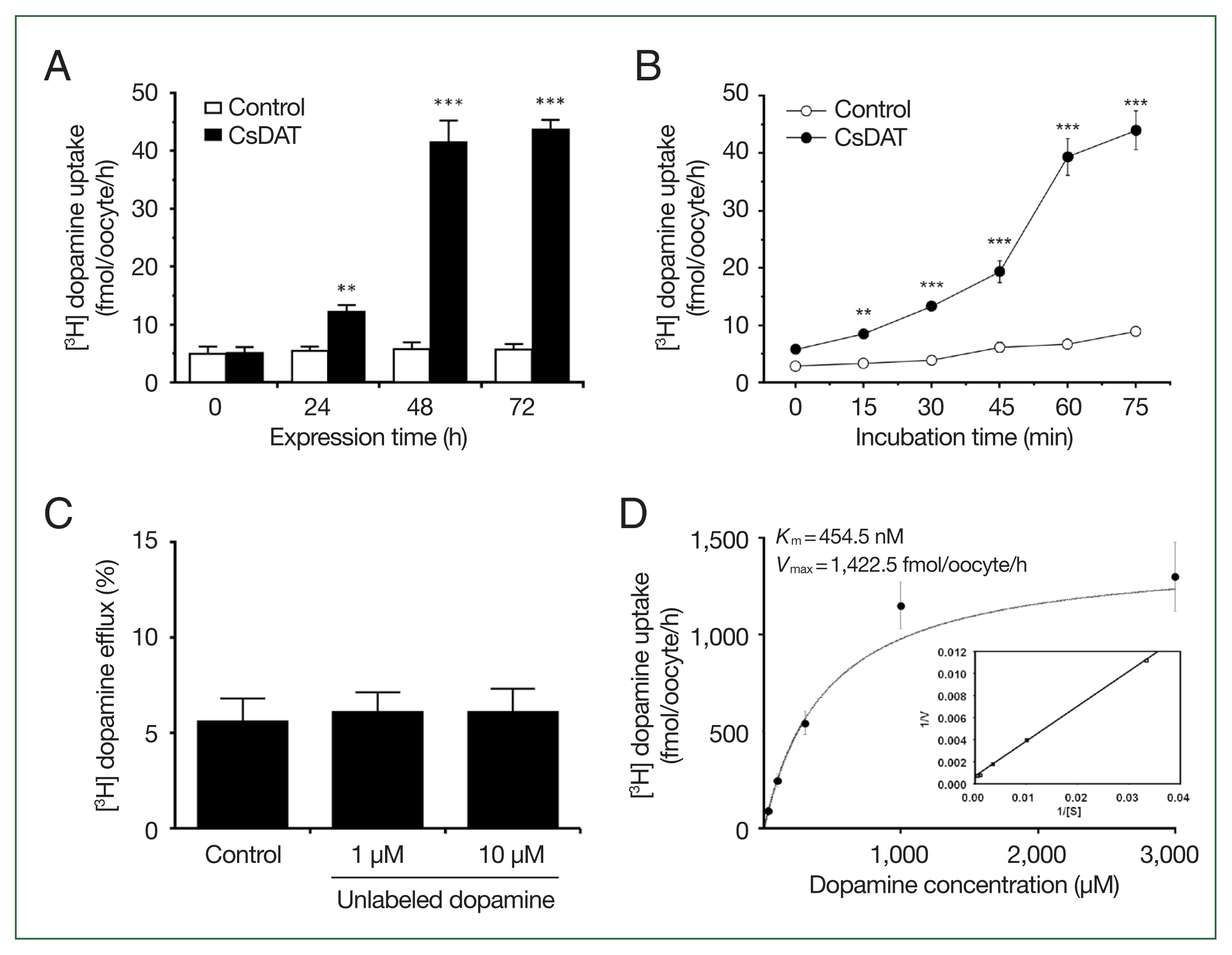
Dopamine transport properties of CsDAT. (A) The time course of [3H] dopamine uptake in CsDAT-expressing Xenopus laevis oocytes over 24–72 h after injection. (B) The time-dependent uptake of [3H] dopamine by CsDAT-expressing oocytes over incubation times ranging from 15 to 75 min. (C) Dopamine efflux assay. Oocytes were preloaded with 30 nM of [3H] dopamine for 90 min and incubated with or without excess unlabeled dopamine (1 μM and 10 μM) for 60 min to assess the substrate-induced efflux. (D) The saturation of CsDAT-mediated uptake of [3H] dopamine. The uptake rates of [3H] dopamine by the control (water-injected) or CsDAT-expressing oocytes for 60 min were measured at variable concentrations. Inset: Eadie-Hofstee plot of the concentration-dependent uptake of [3H] dopamine. V, velocity; [S], dopamine concentration. All results are presented as mean±SE (n=6–8). **P<0.01, ***P<0.001.
Sodium dependence and structural basis of Na+ binding in CsDAT
The Na+ in the standard uptake buffer (ND96) was replaced with either choline or lithium to assess sodium dependence (Fig. 4A). Dopamine uptake was almost completely abolished under both conditions, suggesting that CsDAT is strictly sodium-dependent. Within the tertiary structure, the Na+ binding pocket was identified at residues Gly51, Ile54, Leu399, Asp402, and Ser403 (Fig. 4B), which residues stably formed polar interactions with Na+ at distances of less than 3.5 Å. The binding pocket was located near the dopamine-binding site, suggesting that Na+ binding induces structural modifications that facilitate dopamine binding.
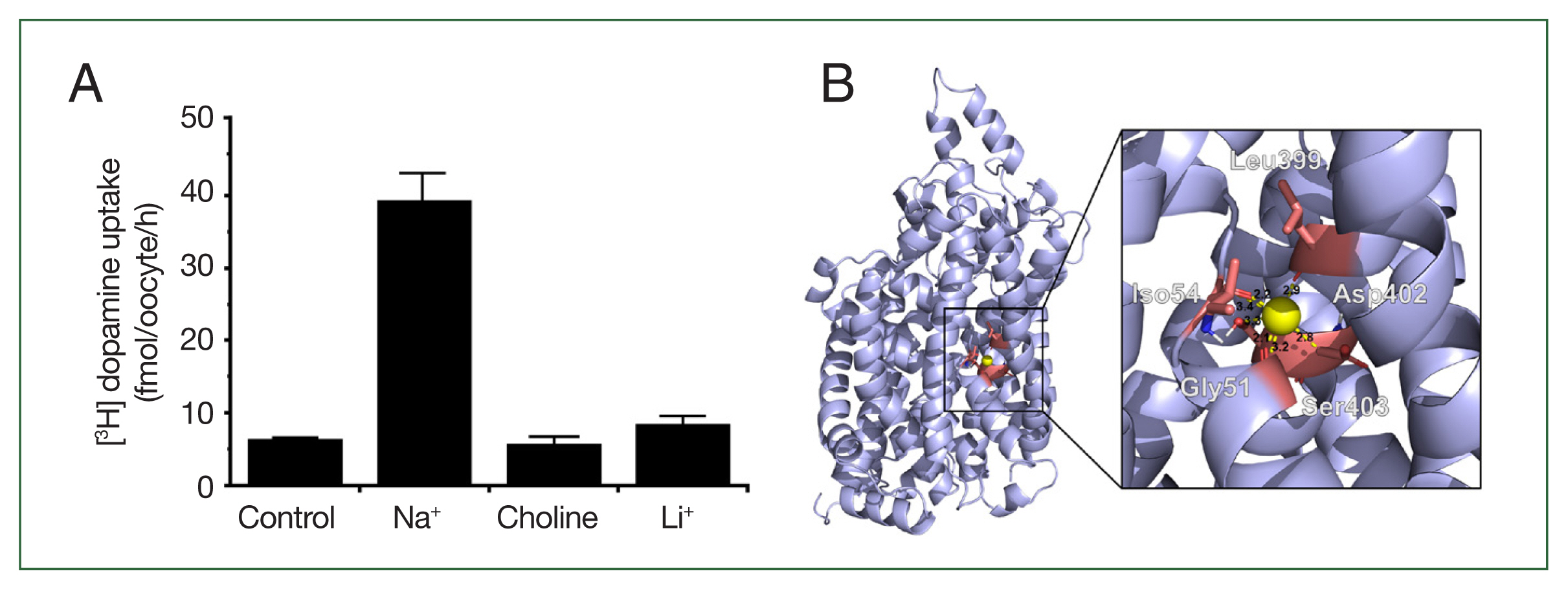
CsDAT sodium dependency. (A) Replacement of extracellular Na+ with choline or Li+ abolished dopamine uptake, demonstrating that CsDAT is sodium-dependent. (B) Sodium-binding residues within the CsDAT pocket. The sodium binding involved Gly51, Iso54, Leu399, Asp402, and Ser403 with a molecular distance of less than 3.5 Å.
Dopamine and inhibitor-binding mode in CsDAT and hDAT
Molecular docking simulations were performed to investigate the binding profiles of dopamine and known inhibitors in CsDAT and hDAT (Fig. 5A). Dopamine was predicted to bind to a conserved pocket in both transporters. In CsDAT, the key residues involved in dopamine binding included Tyr463 and Trp572, which formed hydrophobic interactions, and Arg462, Gly466, Ala467, Asp527, Thr529, and Thr575, which formed hydrogen bonds (Fig. 5B; Supplementary Table S3). Trp84 and Arg85 contributed to hydrophobic interactions in hDAT, while Asp385 and Gly386 were involved in hydrogen bonding (Fig. 5B; Supplementary Table S3).
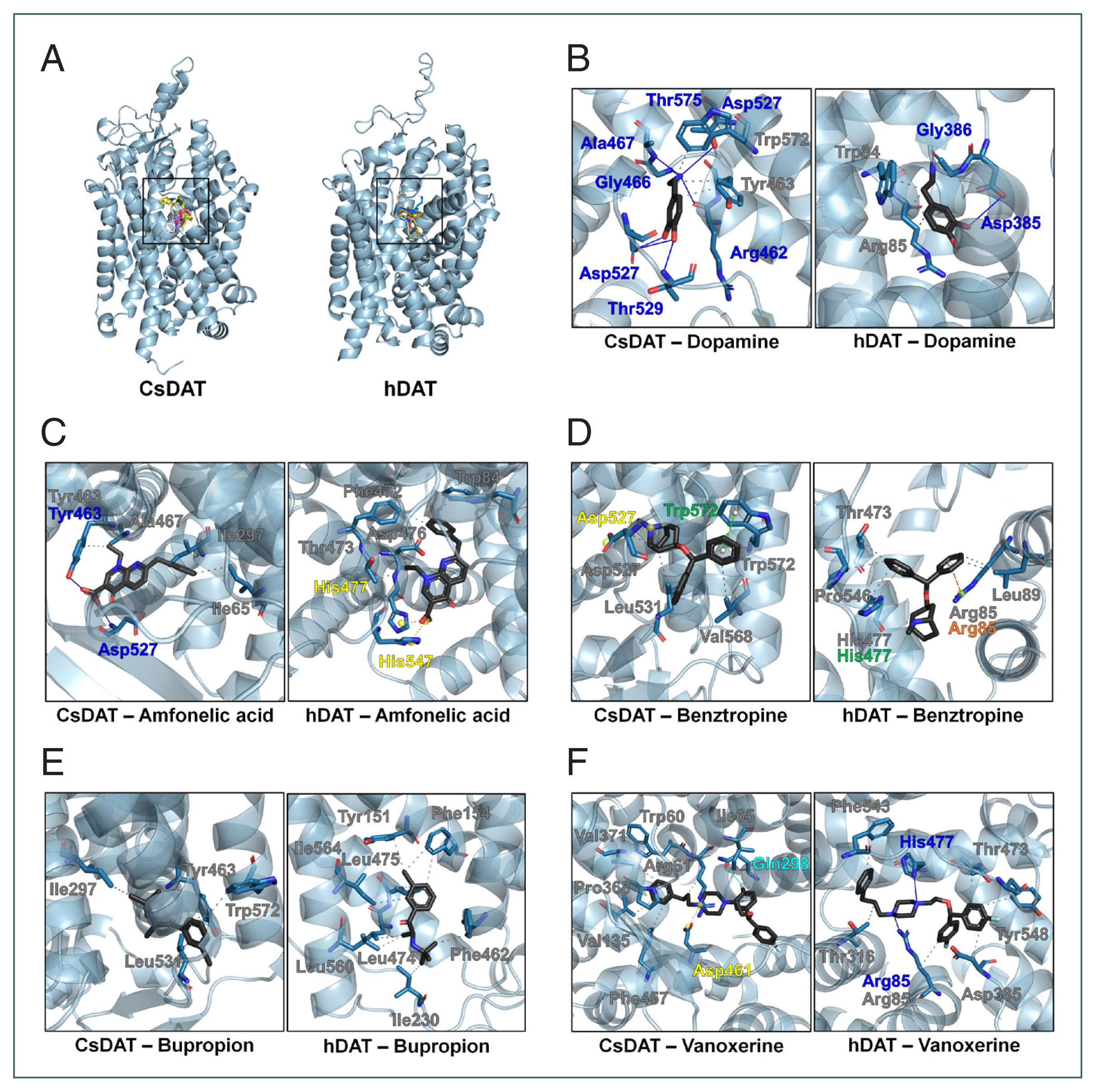
Molecular docking analysis of dopamine and inhibitors binding to CsDAT and hDAT. (A) Overview of the binding pocket for dopamine and inhibitors, including amfonelic acid, benztropine, bupropion, and vanoxerine, in CsDAT and hDAT. (B) Detailed binding interactions of dopamine within the dopamine-binding pockets of CsDAT and hDAT. The key residues involved in hydrophobic interactions (gray) and hydrogen bonding (blue) are indicated. (C–F) Predicted binding poses and interaction residues of the dopamine transporter inhibitors amfonelic acid (C), benztropine (D), bupropion (E), and vanoxerine (F) in CsDAT and hDAT. The intewraction types are color-coded: gray, hydrophobic interactions; blue, hydrogen bonds; yellow, salt bridges; green, Pi-stacking; orange, Pi-cation interactions; cyan, halogen bonds.
The predicted binding affinity of CsDAT for dopamine was −5.5 kcal/mol, which was slightly stronger than that of hDAT (−5.2 kcal/mol) (Supplementary Table S3). In addition to dopamine, docking was performed with 4 known DAT inhibitors (amfonelic acid, benztropine, bupropion, and vanoxerine) to compare their binding affinities and interaction residues between CsDAT and hDAT (Fig. 5C–F; Supplementary Table S3). Supplementary Table S3 provides detailed information on the binding affinities and specific interacting residues. Vanoxerine exhibited the strongest inhibitor binding among all tested inhibitors. The binding affinities of CsDAT were consistently higher than those of hDAT, suggesting potential structural differences that could influence the accessibility or stability of dopamine and its inhibitors within the binding pocket.
Discussion
Dopamine signaling plays a fundamental role in the biology of parasitic helminths, including trematodes, where it regulates neuromuscular activity, osmoregulation, and energy metabolism [8,19,20]. Previous studies in species such as S. mansoni have demonstrated the involvement of DATs in maintaining these essential physiological functions, highlighting their potential as therapeutic targets [17]. However, despite their medical significance as the causative agent of clonorchiasis, little is known about the functional characteristics of DATs in liver flukes.
In this study, we characterized CsDAT (GAA29481), an annotated sodium-dependent DAT from C. sinensis, and demonstrated its functional capacity for dopamine uptake. CsDAT showed high substrate selectivity for dopamine, with a Km value of 454.5 nM, indicating strong affinity and supporting its role in dopamine homeostasis. This function is consistent with that of DATs from other parasitic helminths, such as S. mansoni (Km= 2.0±0.3 μM), but with a significantly higher apparent affinity [21]. Notably, the predominant monoamine neurotransmitters of trematode parasites vary [7]. For instance, serotonin is the dominant monoamine in S. mansoni [22,23], whereas dopamine is predominant in F. hepatica [22,24]. Thus, the high dopamine transport efficiency of CsDAT may reflect species-specific adaptations in dopaminergic signaling among trematodes.
In humans, hDAT is highly expressed in the brain and plays a central role in maintaining dopamine homeostasis, which is crucial for regulating various neurological functions [25,26]. The substrate specificity of hDAT primarily includes dopamine (Km=4.2±0.5 μM), but under certain conditions, it also exhibits a limited capacity to transport other monoamines, such as serotonin [21]. The specificity of CsDAT raises interesting questions about its potential role in the internal processes of parasites. This function could be critical for the parasite’s survival, potentially influencing processes such as motility, feeding behavior, or other metabolic functions that require precise dopamine regulation [27].
Our structural modeling and in silico docking simulations suggest that CsDAT (−5.5 kcal/mol) binds to dopamine with an affinity comparable to that of hDAT (−5.3 kcal/mol), raising the possibility that certain compounds may interact with CsDAT. Given this similarity, it remains to be investigated whether known DAT inhibitors developed for human neurological conditions could show any binding potential toward CsDAT. Compounds such as bupropion, vanoxerine (GBR12909), amfonelic acid, and benztropine are well-characterized inhibitors of mammalian DATs and have shown varying degrees of efficacy against parasite monoamine transporters [21,28,29]. For instance, vanoxerine (GBR12909) is a selective hDAT blocker with high binding affinity, whereas bupropion, an FDA-approved antidepressant [30], exhibits moderate DAT selectivity [31]. These molecules may provide a useful basis for further exploration of their possible relevance to CsDAT. Thus, future in vitro and in vivo studies are warranted to assess their specificity and functional effects on dopamine transport in liver flukes.
The results of the present study demonstrate that CsDAT is a high-affinity DAT that likely plays an important role in maintaining dopamine homeostasis in C. sinensis. Comparing it with DATs from other species reveals both conserved and divergent functional properties, which may reflect ecological and physiological differences. The pharmacological tractability of DATs suggests that CsDAT may be of interest in the context of anthelmintic research. However, further studies are required to evaluate its potential significance and elucidate its roles in parasite biology and host-parasite interactions.
Notes
Author contributions
Conceptualization: Han JH, Cha SH
Data curation: Lee WJ, Kim SJ, Lee WK, Han JH, Cha SH
Formal analysis: Lee WJ, Kim SJ, Lee WK, Han JH, Cha SH
Funding acquisition: Han JH, Cha SH
Investigation: Lee WJ, Cha SH
Methodology: Cha SH
Resources: Cha SH
Software: Lee WJ, Lee WK, Han JH
Supervision: Han JH, Cha SH
Validation: Lee WJ, Kim SJ, Han JH, Cha SH
Visualization: Lee WJ, Cha SH
Writing – original draft: Lee WJ, Han JH
Writing – review & editing: Han JH, Cha SH
Conflict of interest
Jin-Hee Han serves as an editor of Parasites, Hosts and Diseases but had no involvement in the decision to publish this article. No other potential conflicts of interest relevant to this study were reported.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00240627) (JHH) and the National Research Foundation of Korea (NRF-2017R1D1A1B03034588) (SHC). Adult X. laevis were obtained from the Korean Xenopus Resource Center for Research.
Supplementary Information
Supplementary material is available with this article at https://doi.org/10.3347/PHD.25040.
