Fasciola hepatica infection in Korean water deer (Hydropotes inermis argyropus)
Article information
Abstract
Fasciola hepatica is a species of zoonotic liver flukes with a broad range of definitive hosts worldwide. However, this liver fluke has not been detected in Korean water deer (Hydropotes inermis argyropus). This study provides the first evidence for Korean water deer being a definitive host of F. hepatica.
Introduction
Korean water deer (Hydropotes inermis argyropus) are the most dominant wildlife species inhabiting the Korean Peninsula in high densities [1]. They belong to the family Cervidae and are distinguished from other species by their long canine teeth and absence of antlers [2]. Korean water deer prefer dense forest and early successional vegetation and are therefore commonly found in lowland mountains areas or riparian habitats [3]. Their population remains high due to the lack of natural predators in Korea. Korean water deer are frequently observed around farmlands, and they lead to vehicle collisions and crop damage owing to food scarcity and habitat loss [3].
Korean water deer have been suggested as a reservoir for several pathogens that impact domestic animals and humans. There are a few reports about viral, bacterial, and parasitic pathogens detected in Korean water deer. In particular, they are well-known hosts for ticks and potential carriers of tick-borne pathogens such as severe fever with thrombocytopenia syndrome virus [4], Anaplasma spp., Coxiella burnetii, and Babesia capreoli [5]. In addition to the protozoa transmitted through the fecal-oral and water-borne routes such as Sarcocystis [6] and Blastocystis [7], Korean water deer also can have several gastrointestinal parasites [8]. However, Fasciola hepatica infection has not been reported in these deer, although its infections have commonly occurred in various species of domestic animals, such as cattle and sheep, and other wildlife species, such as nutria, in Korea [9,10]. Therefore, in this study, we report the first cases of F. hepatica infection in Korean water deer.
Case Description
From 2017 to 2023, a total of 200 dead Korean water deer were submitted to our laboratory for postmortem examination. At necropsy, large nodular lesions of the liver were observed in 4 of the 200 water deer. Their livers appeared pale with focal fibrotic lesions (Fig. 1A). On the cut surface of the fibrotic tissue, numerous worms formed colonies and were surrounded by white connective tissue (Fig. 1B, C). The worms were dorsoventrally flattened and leaf-like with prominent shoulders and a conical projection. They were 2.5–4.0 cm in length and 0.8–1.0 cm in width, and had highly branched glands on both sides of the body (Fig. 1D).
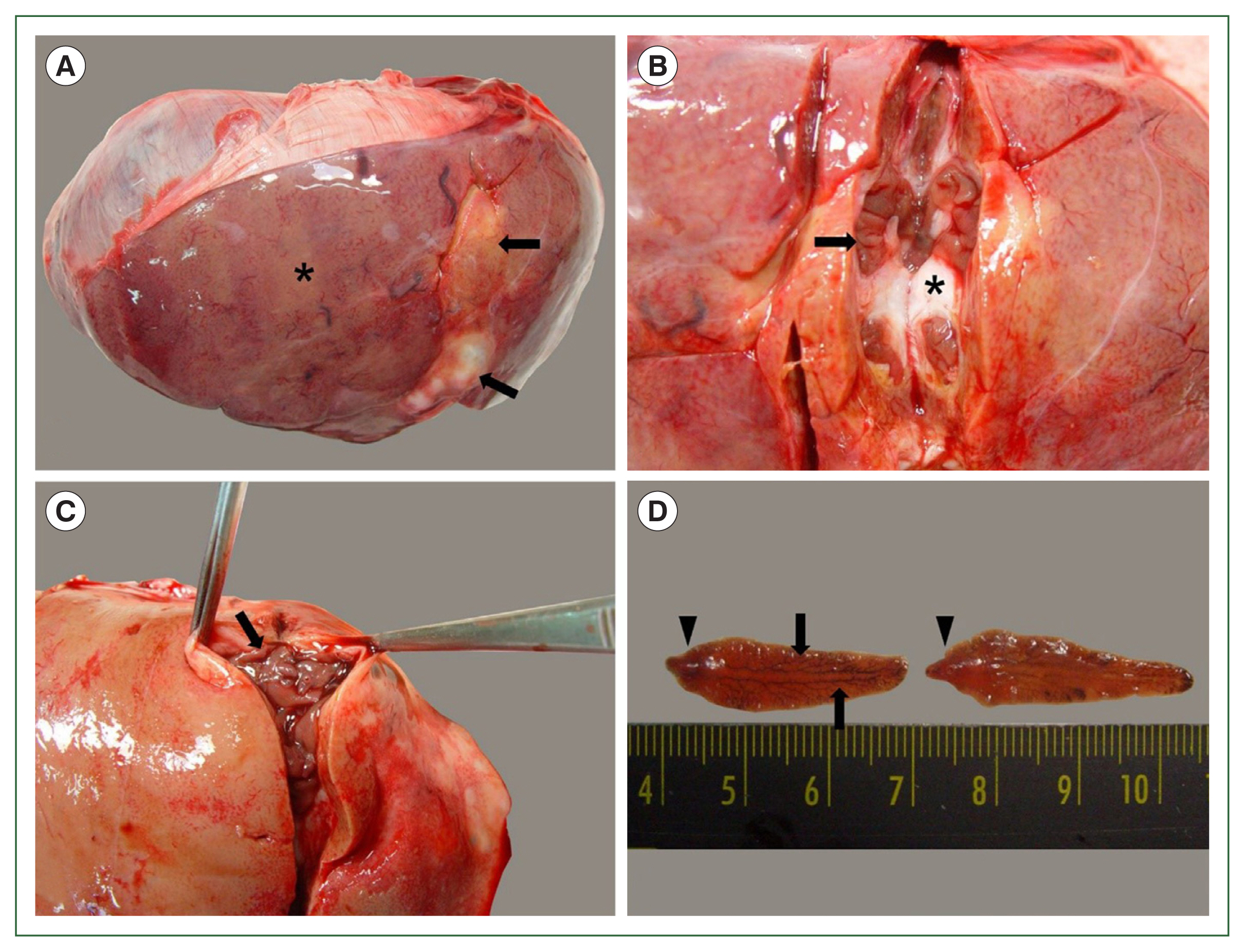
Gross findings of the livers infected with flukes and worms isolated from the liver of water deer. (A) Pale gray discoloration (asterisk) and focal fibrotic lesions (arrows) are observed on the liver surface. (B) On the cut surface of the fibrotic lesion, the worms (arrow) are settled in the liver parenchyma and surrounded by white connective tissue (asterisk). (C) Note the numerous worms burrowing in the hepatic parenchyma. (D) The worms found in the liver. Note the distinct shoulders (arrowheads) behind a conical anterior end, and the highly branched glands (arrows) present on both sides of the body.
For histopathological examinations, liver tissues and worm samples were fixed in 10% formalin, routinely processed, and embedded in paraffin wax. They were sectioned at 4 μm, and subsequently stained with hematoxylin and eosin.
Microscopically, a large worm was detected within the fibrotic lesion, accompanied by the infiltration of inflammatory cells (Fig. 2A). The worm had intestines, vitelline glands, and testes containing mature sperms, and its body was covered by a tegument with sharp cuticular spines (Fig. 2B). Brownish eggs were embedded in the hepatic parenchyma, with surrounding eosinophil infiltration and fibrosis accompanied by hepatocyte atrophy (Fig. 2C). The epithelial cells of the bile duct were hyperplastic within the lesion with severe fibrosis (Fig. 2D). Based on gross and histopathological examinations, the 4 water deer were diagnosed with F. hepatica infection.
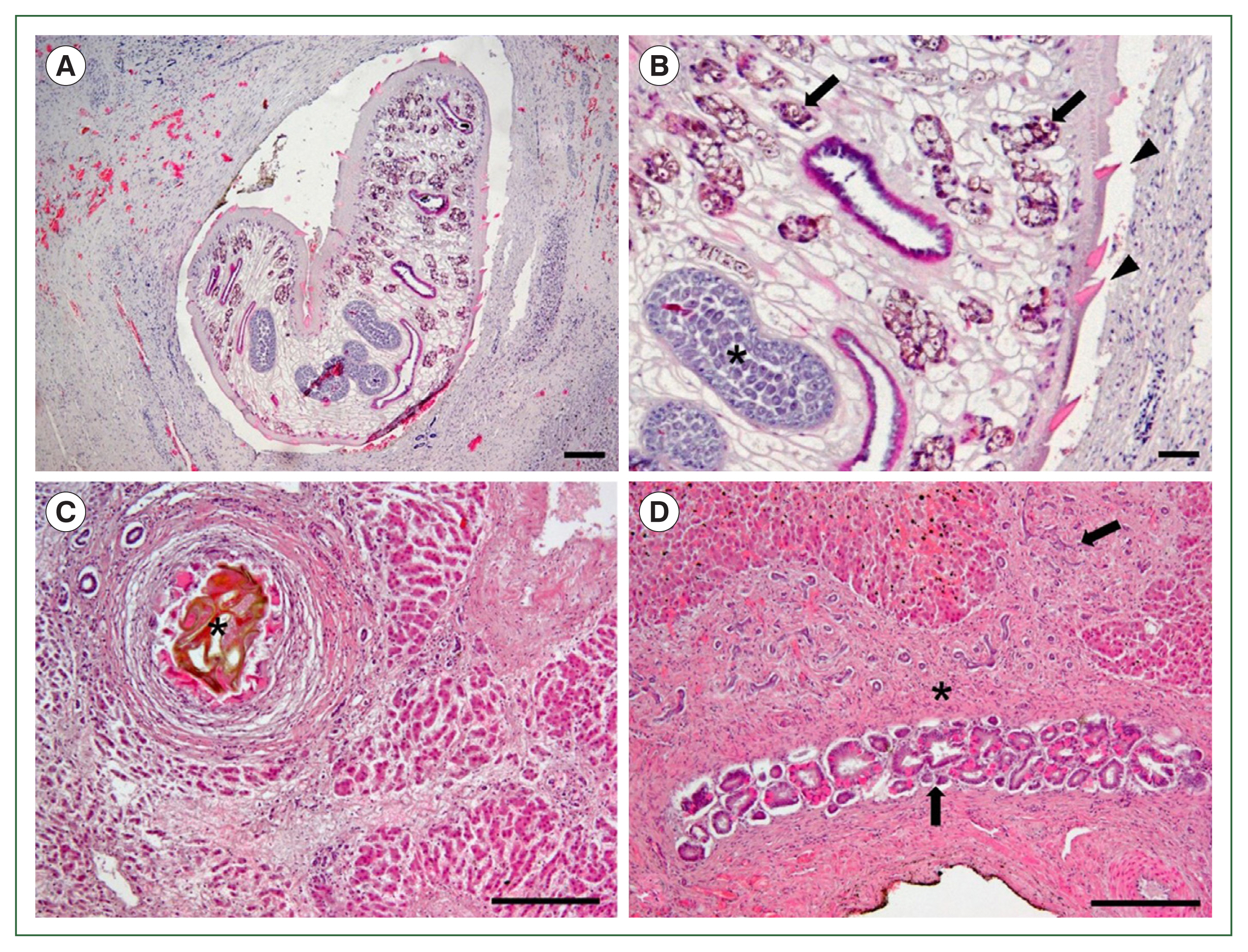
Histopathological findings of the liver infected with liver flukes, Fasciola hepatica. (A) A section of an adult worm within the hepatic parenchyma. The worm is surrounded by fibrotic tissue with inflammatory cell infiltration. (B) Vitelline glands (arrows), testes (asterisk) within the spongy parenchyma, and tegumental spines (arrowheads) covering the body of the worm are noted. (C) Brownish eggs (asterisk) embedded in the hepatic parenchyma. The eggs are surrounded by eosinophil infiltration and fibrosis, with hepatocyte atrophy. (D) Hyperplasia of bile duct epithelial cells (arrows) and liver fibrosis (asterisk). H&E. Scale bar=200 μm.
Discussion
Liver flukes are digenetic trematodes classified into the families Opisthorchiidae, Fasciolidae, and Dicrocoeliidae. These liver flukes share similar morphological features; the adult worm body is flattened leaf-like in shape, with 2 suckers for adhesion to the host organ surfaces and for feeding [11,12]. They have an indirect lifecycle that involves 1 or 2 different intermediate hosts such as snails, freshwater fish, and shrimp [13]. Definitive hosts are infected by ingesting water, raw aquatic plants, or intermediate hosts contaminated with the infectious metacercariae of the parasite. These hosts include various animal species, such as herbivorous mammals, horses, pigs, hares, dogs, cats, and rats, and even humans. The eggs produced by adult flukes are passed into the intestine with bile juices and then excreted in the host feces [6,11,14]. In the present case, Korean water deer are expected to be infected by accidental ingestion of water or plants contaminated with metacercariae, owing to their habitat preference for wetlands and streams.
Liver flukes dwell in the liver parenchyma, biliary duct, and gallbladder of vertebrates [12,14]. The migration through the liver parenchyma of the flukes causes considerable tissue damage followed by hemorrhage and fibrosis [11]. Moreover, adult flukes settling in bile ducts may cause mechanical obstruction of the bile ducts, resulting in cholestasis and cholelithiasis [15]. In the present case, as the number of worms increased in the bile duct, the main parasitic area, it is hypothesized that adult flukes stimulated the tissue and caused fibrosis while migrating through the hepatic parenchyma in the Korean water deer, which lack a gallbladder.
Fasciola spp. are one of the major zoonotic trematodes worldwide that parasitize the livers of particular herbivorous mammals, especially ruminants. Fasciola spp. can be distinguished from other adult fluke worms by the presence of an extensively developed vitelline gland and branched testes located in the medial portion [10,12,14]. Fasciola spp. include 2 species: F. hepatica and Fasciola gigantica, which differ in geographic distribution and morphological features [16]. F. hepatica is distributed worldwide, measuring approximately 1.8–5.0 cm in length and 1.0 cm in width, with a clear shoulder. In contrast, F. gigantica exists in a narrower distributional range across Asia and Africa, measuring up to 7.5 cm in length and 1.2 cm in width, with a less prominent shoulder [11]. Additionally, these 2 species can be differentiated through molecular techniques such as PCR analysis targeting the ribosomal internal transcribed spacer 1 region [17]. In the present case, based on the histopathological and morphological characteristics of the worms found in the liver, the Korean water deer were diagnosed with F. hepatica infection.
In Korea, the prevalence of F. hepatica infection has been confirmed in freshwater snails as intermediate hosts [18] and in cattle, humans, and nutrias as definitive hosts [10]. Given that Korean water deer inhabit waterside environments and that F. hepatica infections have been reported in various deer species in other countries [19], the probability of F. hepatica infection in Korean water deer was initially considered high. However, in this study, F. hepatica was detected in only 2% (4 out of 200) of the Korean water deer, indicating a relatively low incidence rate. Although the number of water deer included in this study may not fully represent the entire population, this low infection rate could explain why F. hepatica had not been identified in Korean water deer before this study.
Although it has been well known that Korean water deer act as reservoirs of tick-borne pathogens [5], there have been no reports of helminthic infections until now. This report describes the first cases of F. hepatica infection in Korean water deer, providing evidence that this species can serve as a definitive host of liver flukes.
Notes
Author contributions
Conceptualization: Hong IH
Data curation: Jeon YJ, Jung IJ
Funding acquisition: Kim B
Investigation: Kim NH
Project administration: Jeon YJ, Jung IJ
Supervision: Hong IH
Validation: Seo MG
Writing – original draft: Kim NH
Writing – review & editing: Seo MG, Hong IH
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
This work was supported by a grant from the National Institute of Wildlife Disease Control and Prevention (NIWDC), and funded by the Ministry of Environment (MOE) of the Republic of Korea (NIWDC-2023-SP-2023-011).
