Glucose-6-phosphate dehydrogenase variants in Kachin, Myanmar
Article information
Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an X-linked recessive genetic disorder that can cause severe anemia in affected individuals exposed to oxidative stress. This risk is particularly relevant in patients treated with the antimalarial drug primaquine. In Myanmar, primaquine has been widely administered as a Plasmodium vivax malaria treatment; however, prevalence of G6PD deficiency among the population remains insufficiently characterized. This study investigated the prevalence of G6PD variants among various minority ethnic subgroups residing in Kachin State, Myanmar. Blood samples from 440 participants were analyzed; however, the Mahidol variant (G487A) was identified in 21 individuals (4.8%). A major limitation of this study was the absence of G6PD enzyme activity data to confirm whether the Mahidol variant induces G6PD deficiency.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an X-linked recessive genetic disorder that is characterized by an inherited enzyme deficiency that can cause hemolysis [1]. First described in 1956 in patients who experienced hemolytic crisis following primaquine administration [2], G6PD deficiency currently affects approximately 500 million individuals globally, with over 400 human G6PD mutations identified [3]. If undiagnosed and untreated, G6PD deficiency can potentially lead to oxidative damage, hemolysis, severe hyperbilirubinemia, bilirubin-induced neurologic dysfunction, acute bilirubin encephalopathy, and kernicterus [4]. Understanding the prevalence and distribution of G6PD mutations and enzyme deficiency is crucial for public health.
The World Health Organization recommends measuring G6PD activity before initiating radical malaria treatment, particularly when using primaquine [5]. Plasmodium vivax can remain dormant in the liver and trigger recurrent infections unless treated with gametocidal or radical-cure therapies such as 8-aminoquinoline drugs, including primaquine. Since primaquine can cause severe anemia in patients with P. vivax malaria who also have G6PD deficiency, the screening and evaluation of G6PD deficiencies should be implemented to ensure the safe treatment of P. vivax infections [6].
P. vivax is predominantly located in Kachin State, an area situated in the northernmost region of Myanmar along the China and Myanmar border. Currently, the most significant malaria hotspot along the China and Myanmar border is the region that includes Laiza and nearby areas in Kachin Special Region II [7]. Malaria cases in Laiza and nearby areas, Kachin State, surged to 8,356 in 2023, indicating an increase of 8,129 cases from 2019 (227 cases) [8]. Considering that primaquine is the first-line treatment for P. vivax in Myanmar [9], research on the prevalence of G6PD mutations, which may lead to G6PD enzyme deficiency, is important to develop effective malaria treatment strategies. Thus, this study aimed to assess the prevalence of G6PD mutations in healthy participants from various minority ethnic subgroups in the Myitkyina and Wai Maw townships, which are located within the Myitkyina District of Kachin State, Myanmar (Fig. 1).
The map of the Myanmar blood collection regions: the Myitkyina and Wai Maw townships in Kachin State.
This study collected a total of 440 blood samples from a healthy population in 2019. Ethical approval for this study was granted by the Bioethics Committee of Kyungpook National University (2019-0042) and the Institutional Review Board of the Department of Medical Research, Ministry of Health and Sports, Myanmar (Ethics/DMR/2019/131). All the study participants provided written informed consent before participating in the study, including permission to collect their blood samples. The participants varied by sex, with 159 males (36.1%) and 281 females (63.9%), age (5–96 years), and ethnic groups, including 219 Jinghpaw, 31 Zaiwa, 35 Lhaovo, 46 Lachid, 13 Lisu, 92 Rawang, 2 Chin, 1 Shan, and 1 Rakhine.
The genomic DNA extraction and PCR amplification for exons 3–4, 6–7, and 9 in the G6PD were performed as previously described [10]. These exons were selected based on a previous study that identified major mutations associated with G6PD deficiency [11]. Subsequently, the PCR products were sequenced, and the resulting nucleotide sequences were aligned with the reference G6PD sequence (accession No. X55448) using Clustal Omega. Of the 440 tested individuals, the Mahidol variant (G487A) was observed in 21 (4.8%) individuals. The Mahidol variant was the most commonly reported in Myanmar, despite having a difference in frequency [12–14]. The World Health Organization classifies the Mahidol variant as a Class III G6PD deficiency, which is characterized by a mild deficiency in the enzyme. Interestingly, other previously reported variants in Myanmar, especially along the China and Myanmar border, such as those in Kachin State, including Chinese-4 and Viangchan, were not observed in the present study [15].
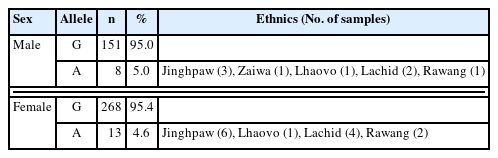
The Mahidol variant was more common in males (5.0%, 8/159) than in females (4.6%, 13/281) (Table 1). Since G6PD deficiency is an X-linked hereditary disease, this deficiency typically affects males more frequently than females [16]. Moreover, previous studies have suggested that certain G6PD variants, such as the Mahidol variant in males, may exhibit protective effects against severe malaria [17]. However, it is difficult to confirm the relationship between malaria susceptibility, G6PD enzyme activity, and the Mahidol variant based on the results of this study, as G6PD enzyme activity was not measured.
Previous research has focused primarily on Burmese, Kachin, and Rakhine ethnicities, where the Mahidol variant predominates [12,14,17,18]. Other variants—Kaiping, Viangchan, Chinese, Mediterranean, Union, Canton, and Orissa—have been reported, with distribution patterns varying geographically [10,12,17,19]. However, the prevalence of G6PD variants in the minor ethnic subgroups has not been assessed. In the ethnic groups studied in this research, the highest prevalence of the Mahidol variant was identified in Lachid (13.0%, 6/46), followed by Lhaovo (5.7%, 2/35), Jinghpaw (4.1%, 9/219), Rawang (3.3%, 3/92), and Zaiwa (3.2%, 1/31) (Table 1).
In conclusion, the Mahidol variant was identified across multiple minority ethnic groups residing in Kachin State, Myanmar. These findings on the prevalence of G6PD variants and their relationship with malaria susceptibility provide valuable baseline information, emphasizing the importance of G6PD screening before malaria treatment.
Notes
Author contributions
Conceptualization: Goo YK
Data curation: Lee S, Goo YK
Formal analysis: Moon Z, Lee S
Investigation: Moon Z, Aung JM, Yun HS, Dinzouna-Boutamba SD
Resources: VanBik D, Yun HS, Ring Z, Chung DI
Supervision: Hong Y, Chung DI, Goo YK
Validation: Goo YK
Writing – original draft: Goo YK
Writing – review & editing: Hong Y
Conflict of interest
Yeonchul Hong and Youn-Kyoung Goo serve as editors of Parasites, Hosts and Diseases but had no involvement in the decision to publish this article. No other potential conflicts of interest relevant to this study were reported.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2024, and we thank all participants for donating blood samples and the field staff for their assistance during the survey process.