Strain-dependent immune responses reveal a critical role of IL-17A in defense against Balamuthia mandrillaris
Article information
Abstract
Balamuthia mandrillaris is a causative agent of granulomatous amebic encephalitis, a rare but often fatal condition. To investigate the role of T helper (Th) cell subsets in the immune response against B. mandrillaris, we examined 3 mouse strains with distinct immunological profiles: C57BL/6 (Th1-dominant), BALB/c (Th2-dominant), and ICR (balanced Th1/Th2). Mice were infected intranasally with 1×105 amoebae. Body weight and neurologic symtoms were monitored weekly, and cytokine expression was assessed biweekly over 6 weeks. Minimal weight loss and no mortality were observed in C57BL/6 mice, whereas BALB/c and ICR mice exhibited significant early and delayed mortality, respectively. Interleukin-17A expression was notably elevated in C57BL/6 mice compared with the other strains. These findings indicate that a robust Th17 response, particularly interleukin-17A production, is a critical component of the host defense against B. mandrillaris infection.
Balamuthia mandrillaris is a free-living amoeba that can infect the brain via skin abrasion or nasal inhalation [1]. Infections caused by this organism lead to granulomatous amoebic encephalitis, a rare but often fatal disease characterized by inflammation and the destruction of neural tissue following invasion of the central nervous system (CNS) [2]. Owing to the high observed mortality rate and ability to infect individuals regardless of geographic location or immune status, this pathogen poses a significant global health concern [3]. A study of B. mandrillaris infections in the United States from 1974 to 2016 identified 109 cases, with a mortality rate of 90% [4]. Separately, 28 cases were reported in China between 2000 and 2020 [5]. Although cases of B. mandrillaris infection in Asia are rare, all reported cases have been fatal [6]. Two cases were documented in Korea. The first case, reported in 2019, involved a 71-year-old male with rheumatoid arthritis who was receiving immunosuppressive therapy; the patient died within 10 days of diagnosis [7]. The second case, reported in 2020, involved a 50-year-old immunocompetent male with no prior neurological disorders, who also died within 10 days of diagnosis [8]. These cases highlight that B. mandrillaris infection can affect both immunocompromised and immunocompetent individuals. Previous research on B. mandrillaris infections has employed both immunocompetent and immunodeficient mouse models. In a 1996 study, 7 out of 10 SCID BALB/c mice infected intranasally with 1×106 amoebae succumbed to infection, compared to only 1 out of 10 wild-type BALB/c mice [9]. Similarly, a 2007 study reported higher mortality rates in rag1−/− C57BL/6 mice than in wild-type mice following oral or intranasal infection with 1×104 amoebae [10]. These findings highlight the strong pathogenicity of B. mandrillaris and the critical role of T cell-mediated immune responses in combating infections. For example, the T cell-mediated immune response is an important protection mechanism against the pathogens of Acanthamoeba and Naegleria. Suryawanshi et al. [11] demonstrated that Acanthamoeba infection in mice induces robust T helper (Th)1, Th2, Th17, and regulatory T cell (Treg) responses in the cornea and draining lymph nodes. Interleukin (IL)-17A was found to be critical for host protection, as its neutralization resulted in increased disease severity. Additionally, Naegleria fowleri infection activates both innate and adaptive immune responses, including macrophage and T cell activation. However, the rapid progression of the disease often outpaces the immune response, resulting in high mortality [12]. The specific roles of Th cell subsets (e.g., Th1, Th2, and Th17) in the immune defense against B. mandrillaris remain unclear. Therefore, targeted investigations are needed to elucidate the roles of these subsets in the host defense against B. mandrillaris. In this study, we investigated the role of T cell-mediated immunity in combating B. mandrillaris infection, focusing on the contribution of specific Th cell subsets to the host defense. Using mouse strains with different immune response profiles (Th1-dominant C57BL/6, Th2-dominant BALB/c, and balanced ICR), we aimed to identify T cell responses critical for protection against this pathogen. Our findings provide novel insights into the immune mechanisms underlying host-pathogen interactions.
All experiments were conducted using 8-week-old female C57BL/6, BALB/c, and ICR mice. The animal protocols used in this study were reviewed and approved by the Pusan National University Institutional Animal Care and Use Committee for ethical procedures and scientific care (approval No. PNU-2024-0453). B. mandrillaris (ATCC 50209 strain) was cultured in Eagle’s minimum essential medium (ATCC 30-2003) supplemented with 2 mM L-glutamine, Earle’s balanced salt solution, 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 3% fetal bovine serum (FBS). A monolayer of Vero cells (Korean Cell Line Bank, No. 10081) was used for host cell support. Vero cells were cultured in RPMI 1640 medium containing 300 mg/L L-glutamine, 25 mM HEPES, 25 mM sodium bicarbonate, and 10% heat-inactivated FBS [13]. The cells were incubated for 2–3 days until a confluent monolayer was formed. After the Vero cells adhered to the bottom of the flask, the medium was carefully replaced with RPMI 1640 containing B. mandrillaris to avoid detaching the host cells. To harvest B. mandrillaris, the cysts were detached by gently scraping the bottom of the flask with a sterile cell scraper. The cell suspension was transferred to 50 ml centrifuge tubes and centrifuged at 800 ×g for 5 min. The resulting pellet was resuspended in 1 ml of phosphate-buffered saline. The selection of amoeba inoculum concentration and infection routes was based on previous foundational studies [10]. Briefly, B. mandrillaris was administered to mice via the intranasal route. The mice were anesthetized with isoflurane, and 1×105 amoebae suspended in 10 μl of phosphate-buffered saline were carefully introduced into the nasal cavity. Mice were monitored for 42 days post-infection. Body weight was recorded weekly, and neurological symptoms were observed daily for 6 weeks. Humane endpoints were determined based on health conditions and clinical symptoms, and mice were euthanized accordingly. To measure the concentrations of tumor necrosis factor (TNF)-α, IL-5, and IL-17A, an ELISA was performed on cells isolated from the spleen. Cells were collected from the 3 mouse strains at 2-, 4-, and 6-weeks post-infection. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS at 37°C in 5% CO2 for 3 days. The culture supernatants were analyzed using an ELISA kit (eBioscience, San Diego, CA, USA) and 96-well ELISA plates. The plates were coated with capture antibodies and incubated overnight at 4°C. Nonspecific binding was blocked by incubating the membrane in a 1% bovine serum albumin blocking solution. Standard cytokine samples and culture supernatants were added to the wells, and the plates were incubated overnight at 4°C. After washing, biotin-conjugated and enzyme-linked secondary antibodies were added. The substrate solution was then added, followed by sulfuric acid (H2SO4) to stop the reaction. Finally, absorbance was measured at 450 nm, and cytokine concentrations were calculated based on a standard curve. Statistical analysis was performed using Student t-test in Prism version 6 (GraphPad Prism, La Jolla, CA, USA). Data are presented as the mean±SD. A P-value <0.05 was considered statistically significant.
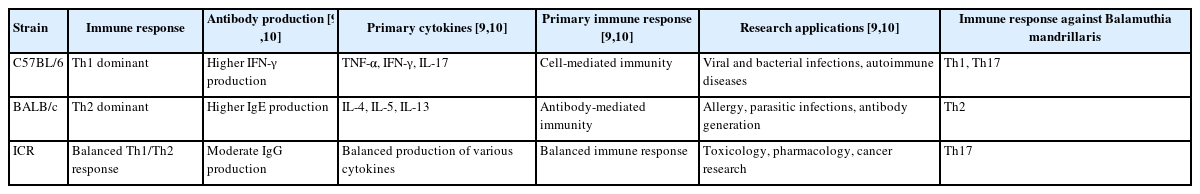
All C57BL/6 strain mice infected via intranasal routes survived (Fig. 1A). Body weights in the infected group remained comparable to those of the control group over the 42-day observation period. In contrast, 3 BALB/c mice in the infection group died on day 14, and their body weights decreased during the early phase of infection (weeks 1 and 2 post-infection) but gradually stabilized in the later stages. ICR mice also exhibited a strain-specific response, with 3 mice dying by day 21. Unlike BALB/c mice, the body weight of ICR mice decreased during the late infection phase (3–4 weeks post-infection) (Fig. 1B, C). TNF-α levels were slightly higher in C57BL/6 mice than in BALB/c or ICR mice, although the difference was not statistically significant, reflecting the Th1-dominant immune profile of this strain (Fig. 2A). IL-5 production was highest in BALB/c mice and was significantly higher than in C57BL/6 (P<0.01) and ICR mice (P<0.001). C57BL/6 mice exhibited minimal IL-5 production, consistent with their Th1-biased responses. ICR mice showed intermediate IL-5 levels, reflecting their balanced Th1/Th2 profile (Fig. 2B). IL-17A levels were highest in C57BL/6 mice, indicating strong Th17 activation. IL-17A levels were lower in BALB/c mice than in C57BL/6 mice, and ICR mice also exhibited low IL-17A levels that were not significantly different from those in BALB/c mice (Fig. 2C). Following infection, TNF-α and IL-5 levels in C57BL/6 and ICR mice were not significantly altered relative to the basal level. However, in BALB/c mice, TNF-α levels were significantly elevated at 2 and 4 (P<0.01), and 6 weeks (P<0.05) post-infection (Fig. 3A). IL-5 levels did not change significantly (Fig. 3B). In C57BL/6 mice, IL-17A levels were significantly increased at 2 and 4 weeks post-infection (P<0.05) (Fig. 3C). BALB/c mice exhibited relatively low IL-17A levels, with no significant changes observed at any time point post-infection (Fig. 3C). In contrast, ICR mice exhibited a significant increase in IL-17A levels at 6 weeks post-infection (P<0.01), although no significant differences were observed at earlier time points (Fig. 3C).
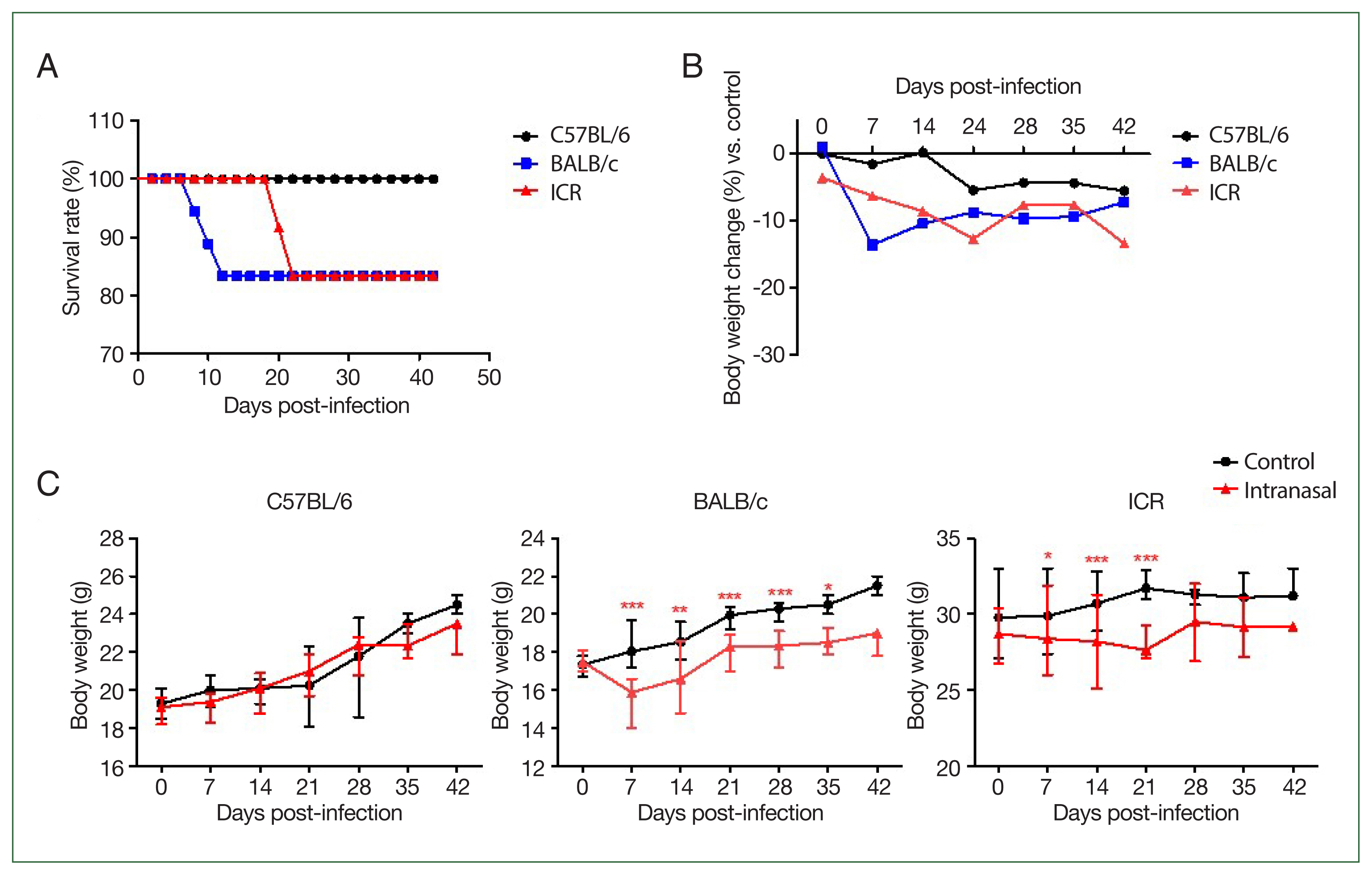
Survival rate and body weight changes in 3 mouse strains following Balamuthia mandrillaris infection. (A) Experimental timeline showing the intranasal infection routes during a 42-day observation period. (B) Comparison of body weights between infected and uninfected control mice at each time point. C57BL/6 mice maintained a relatively stable body weight, whereas BALB/c and ICR mice exhibited sustained weight loss over time. (C) Body weight changes over 42 days post-infection. C57BL/6 mice showed body weight stability throughout the observation period, indicating strong resistance to B. mandrillaris infection. In contrast, BALB/c mice showed weight loss during the early stage, while ICR mice displayed weight loss during the later stages of infection. Statistical significance was determined using an unpaired Student t-test (*P<0.05, **P<0.01, ***P<0.001).
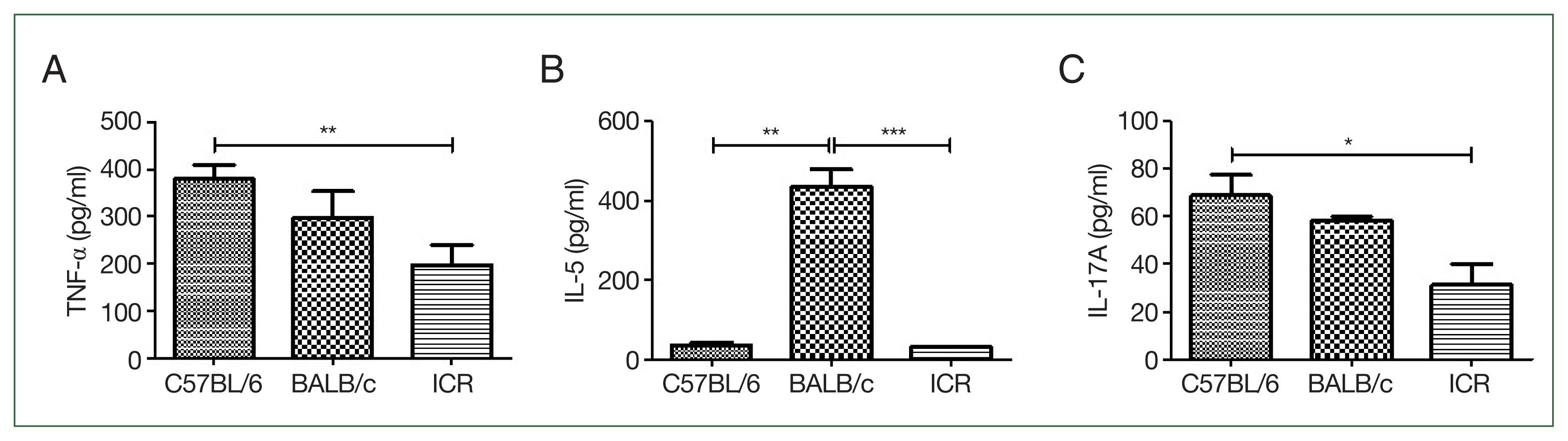
Baseline T helper (Th)1, Th2, and Th17 cytokine profiles of each mouse strain. (A) Baseline levels of tumor necrosis factor (TNF)-α (Th1 cytokine) measured using ELISA. (B) Baseline levels of interleukin (IL)-5 (Th2 cytokine) measured using ELISA. BALB/c mice exhibited significantly higher IL-5 levels, indicating a Th2-dominant response. (C) Baseline levels of IL-17A (Th17 cytokine) measured using ELISA. IL-17A levels were elevated in C57BL/6 mice, reflecting a Th1/Th17-dominant profile. ICR mice displayed moderate cytokine levels, representing a balanced Th1/Th2/Th17 profile. The plates were read at 450 nm. Each P-value was determined using a t-test compared with the control (n=5 mice/group, 3 independent experiments, *P<0.05, **P<0.01, ***P<0.001).
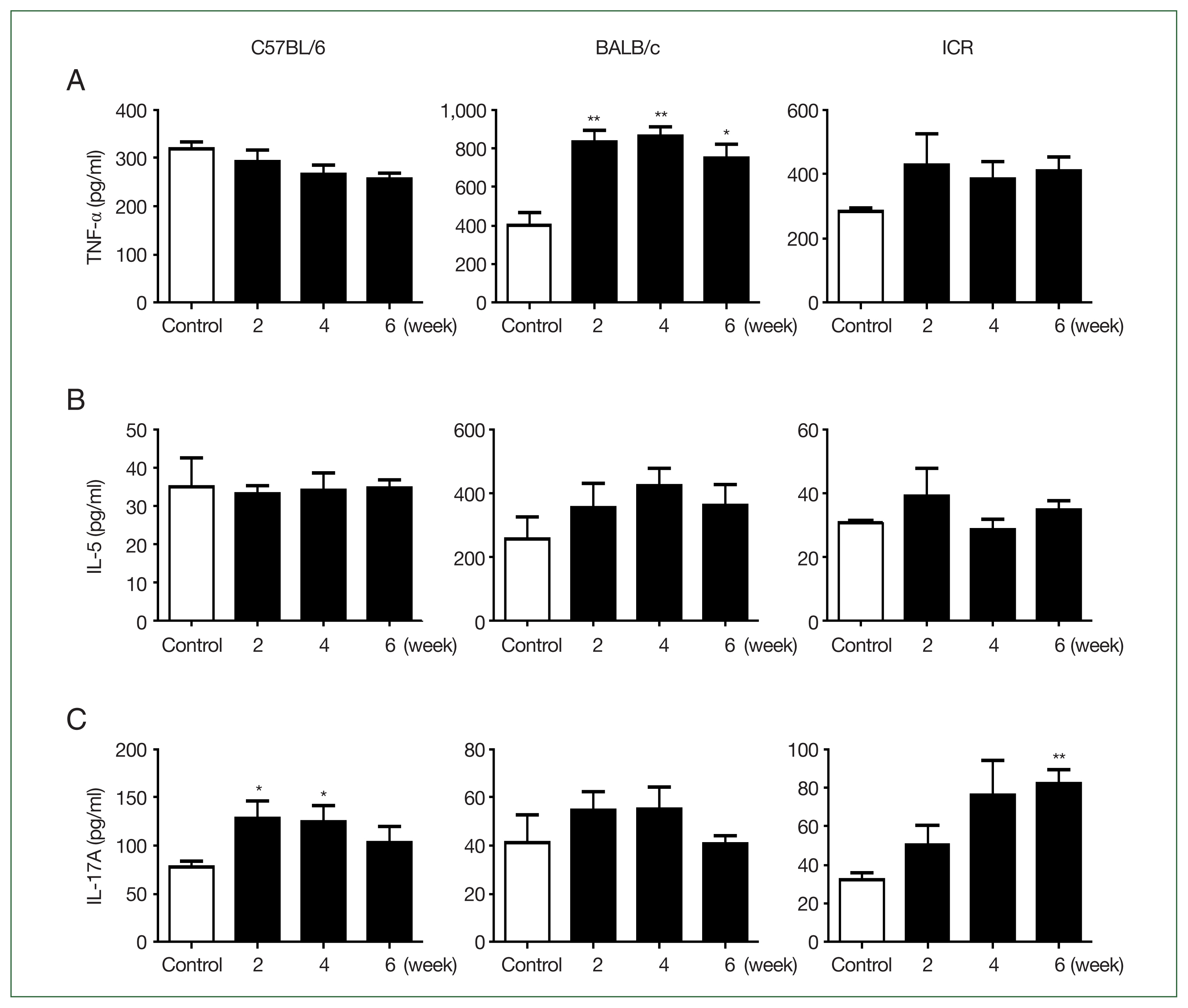
ELISA results for tumor necrosis factor (TNF)-α, interleukin (IL)-5, and IL-17A analyzed in the different mouse strains. (A) TNF-α (T helper (Th)1 cytokine) levels measured using ELISA. BALB/c mice exhibited significantly higher TNF-α levels after infection, indicating the pro-inflammatory response was activated. (B) IL-5 (Th2 cytokine) levels measured using ELISA. BALB/c mice maintained elevated IL-5 levels throughout infection, suggesting a persistent Th2-dominant profile. (C) IL-17A (Th17 cytokine) levels measured using ELISA. In C57BL/6 mice, IL-17A levels were significantly elevated at 2 and 4 weeks post-infection, indicating a sustained Th17 response; in ICR mice, significant increases were observed at 6 weeks. The plates were read at 450 nm. Each P-value was determined using a t-test compared with the control (n=5 mice/group, 3 independent experiments, *P<0.05, **P<0.01).
With the recent exacerbation of global warming, new diseases have been frequently observed in specific regions. Notably, B. mandrillaris infection has been predominantly reported in areas with high average annual temperatures. However, as temperate regions, such as Korea, experiencing a shift toward subtropical climates, new outbreaks have been reported [7]. Detailed immunological studies of the host are therefore essential to control these newly emerging and increasing diseases. B. mandrillaris is a free-living amoeba capable of causing severe brain infections that lead to granulomatous amoebic encephalitis [14]. Despite the high mortality rate, the immune mechanisms underlying resistance to B. mandrillaris infection remain poorly studied [15]. To address this gap, we investigated strain-dependent immune responses to B. mandrillaris infection in 3 mouse strains with different immunological profiles: C57BL/6, BALB/c, and ICR. The survival rate varied significantly, indicating the different susceptibility of each strain to infection. All C57BL/6 mice survived the infection. BALB/c mice died during the early stages (2–3 weeks), and ICR mice died in the later stages (3–4 weeks), indicating strain-dependent resistance (Fig. 2). Thus, the host genetic background and differences in immune response are critical for determining survival outcomes following B. mandrillaris infection. Consistently, IL-17A levels were significantly elevated in C57BL/6 mice at 2 and 4 weeks post-infection (P<0.05), suggesting a sustained Th17 response against B. mandrillaris (Fig. 3C). IL-17A has been shown to enhance the blood-brain barrier integrity and mediate protective neuroinflammatory responses during protozoal brain infections, as observed in Toxoplasma gondii and Trypanosoma brucei encephalitis [16]. In contrast, BALB/c mice exhibited significantly elevated IL-5 levels at baseline, that remained relatively high after infection (Figs. 2B, 3B), indicating a persistent Th2-biased environment. This cytokine profile may suppress effective pro-inflammatory mechanisms by favoring eosinophilic and humoral pathways, which are generally less effective against intracellular or invasive protozoa. Similar findings have been reported in models of T. gondii infection, in which IL-5-dominant environments were correlated with increased parasite burden and mortality [17]. Collectively, our findings suggest that an IL-17A–driven Th17 response may be essential for protective immunity against B. mandrillaris, whereas elevated Th2 cytokines such as IL-5 may impair cellular immune responses and increase susceptibility. Based on our comparative analysis, C57BL/6 mice exhibited a combined Th1 and Th17 response, which was associated with complete survival and effective resistance to infection. In contrast, BALB/c mice, with a Th2-dominant profile characterized by persistently high IL-5 levels, exhibited early mortality and reduced inflammatory efficacy. These findings indicate that Th17 and Th1 responses contribute to protective immunity against B. mandrillaris, whereas a Th2-biased environment may increase susceptibility (Table 1) [9,10]. Despite our clear findings indicating the critical role of Th1 and Th17 cytokines in resistance to B. mandrillaris, the limitations of our study should be considered. Notably, we did not investigate the roles of other important immune components such as Tregs or innate immune cells, including microglia, macrophages, and dendritic cells (DCs), which are known to orchestrate CNS immunity. Tregs, for example, suppress excessive inflammation and limit immunopathology in neuroinfection; however, their overactivation may impair pathogen clearance [18]. DCs are critical for antigen presentation and Th cell polarization in CNS infections [19]. The absence of DC phenotyping and activation profiling limits our understanding of how antigen presentation differs among mouse strains. Future studies should investigate these immune pathways in more depth. We propose transcriptomic profiling of brain-infiltrating immune cells to identify strain-specific expression of cytokine receptors, co-stimulatory molecules, and transcription factors associated with Th subset polarization. Specifically, single-cell RNA sequencing could uncover heterogeneity among T cell subsets and their regulatory networks during pathogen infection [20]. It is likely that B. mandrillaris infection could also be explored in this way. Additionally, in vitro co-culture experiments between DC and naive T cells from different strains could offer functional insights into differential Th17 priming mechanisms. These approaches may lead to new therapeutic avenues for targeting host immune pathways in amoebic encephalitis.
Notes
Author contributions
Conceptualization: Yu HS
Data curation: Han DG, Park MK, Choi SY, Kang SA
Formal analysis: Han DG, Choi SY, Kang SA
Investigation: Jeong YJ, Han DG
Methodology: Jeong YJ, Han DG, Park MK, Choi SY, Kang SA
Supervision: Yu HS
Writing – original draft: Jeong YJ
Writing – review & editing: Kang SA, Yu HS
Conflict of interest
Hak Sun Yu serves as an editor of Parasites, Hosts and Diseases but had no involvement in the decision to publish this article. No other potential conflicts of interest relevant to this study were reported.
Acknowledgments
This work was supported by the the Korea Disease Control and Prevention Agency (KDCA) research project (project No. R25TA00530132-00).