Redescription of Chironomus salinarius (Diptera: Chironomidae), nuisance midges that emerged in brackish water of Jinhae-man (Bay), Kyongsangnam-do, Korea
Article information
Abstract
Huge numbers of non-biting midges emerged from brackish water which were made at the harbor construction field in Jinhae City, Kyongsangnam-do, Korea in late summer in 2005, and caused a serious nuisance to villagers. The midges were collected and identified as Chironomus salinarius (Kieffer, 1921). Although this species was recorded in Korea for the first time in 1998, the morphological descriptions were so brief and simple. A full redescription is made with detailed illustrations for ecological and control workers of this nuisance midge.
INTRODUCTION
During the period of late summer-autumn 2005, a huge number of non-biting midges emerged from brackish water (about 6 km2 in size) in the new harbor construction field in Jinhae City, Kyongsangnam-do, Korea, and caused serious nuisance to humans. Eight males and 10 females, mounted on slides in Hoyer's solution, were identified as Chironomus salinarius (Kieffer, 1921).
Ch. salinarius was reported for the first time in Korea by Chun (1989) in his Master of Science thesis. He described larva, pupa and adult stages of this species. However, his description is too brief and simple, particularly in the adult morphology. Later Yoon and Chun (1992) described this species in English, which were also very brief and incorrect, and simple illustrations were not very helpful for correct identification. Therefore, in this paper, we redescribe the adult morphology of Ch. salinarius in full detail with figures in order to provide useful information to ecological specialists and control workers of this important nuisance midge species.
DESCRIPTION
Chironomus salinarius (Kieffer, 1921) (Fig. 1)
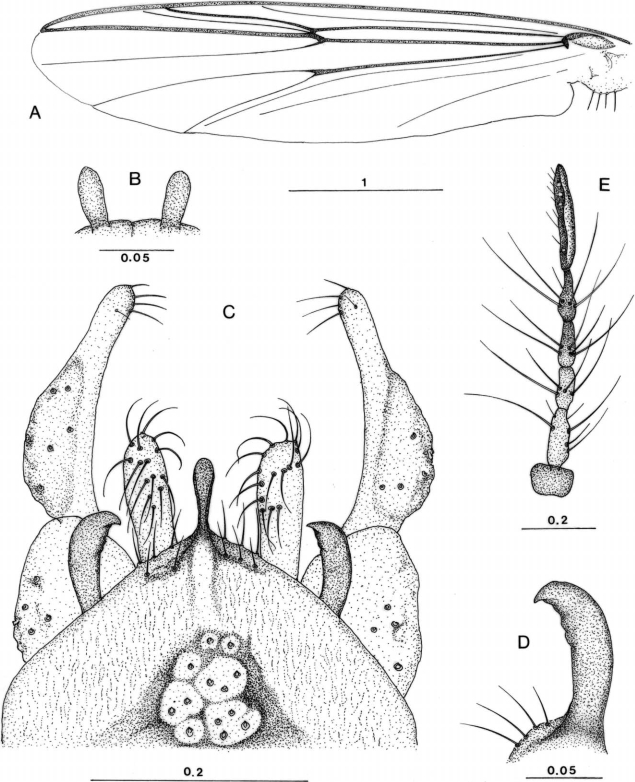
Male of Chironomus salinarius (Kieffer, 1921). A. wing; B. frontal tubercle; C. hypopygium; D. superior volsella; E. antenna of female. Scale: mm.
Chironomus salinarius: Strenzke (1959); Pinder (1978); Sasa (1978); Chun (1989); Yoon and Chun (1992).
Key characters. Mid-size black midge (wing length 3.3 mm in male, 3.8 mm in female). Body almost entirely dark brown. Antennal ratio (AR) 3.5. Leg ratio (LR) 1.4. Superior volsella dark brown, highly chitinized, and strongly curved at tip (like a fishing hook). Gonostylus abruptly narrowed from apical one-third.
Male. Head: compound eye black, bare with well developed dorsal projection. Antenna 12 segmented, AR = 3.5. Palpus 4 segmented: 57, 205, 232, 352 µm (1:3.6:4.1:6.2). Frontal tubercle (Fig. 1B) large (27 µm long and 11 µm wide). Clypeus rectangular with 26 long setae. Thorax: anteropronotum dark brown, with a pair of small setae on middle. Scutum dark brown; central and lateral vittae black, with inconspicuous margin, with 17-18 dorsocentrals each side. Scutellum dark brown, with 25 scutellars. Postnotum black. Halter yellowish at tip. Wing (Fig. 1A): length 3.3 mm. Membrane without macrotrichae. Costa, subcosta, radius (R, R1, R2+3, R3, R4+5) and arculus dark brown; other veins pale. Brachiolum pale brown. Costa not produced. RM oblique, dark brown. An not clearly seen. FCu just under RM. Anal lobe well developed. Squama fringed. Legs: costa dark brown. Femur yellowish brown, with darker basal and apical ends. Tibia yellowish brown with dark brown at basal half and apical tip. Tarsus I yellowish brown. Tarsi II-IV dark brown. Combs of tibia contiguous each with short spur. Pulvilli developed. LR (frontal leg ratio) 1.4. Abdomen: uniformly dark brown, except pale distal margin of tergite I-III and VI-VII. Hypopygium (Fig. 1C): pale brown. Anal point dark brown, round at tip, slightly expanded distally. Apical one third of gonostylus abruptly narrowed, with 1 short apical, 3 long subapical setae internally. Inferior volsella, long, broad, not expanded apically, reaching far beyond tip of anal point, with 15-18 recurved britles. Superior volsella dark brown, bare, chitinized and strongly curved at tip like a fishing hook (Fig. 1D).
Female. All characters, in general, same as in male, with usual sexual differences. Wing length 3.8 mm. Antenna 6 segmented: 84, 166, 107, 129, 135, 310 µm (Fig. 1E).
DISCUSSION
Descriptions of Ch. salinarius in European countries were very brief, so that their characters were not able to compare with the Korean specimens. In Japan, Sasa (1978) described adults of this species in detail. Korean specimens well coincide with Japanese specimens, except the body size; the wing length of the former is shorter than the latter's (3.3 mm vs 4.5 mm in male, 3.8 mm vs 5.4 mm in female). The wing length of England specimens were 3-4 mm (Pinder, 1978). The wing length substitutes for the body size of the non-biting midge, because a measurement of the body length of slide-mounted specimens is not practically possible.
This species is found in Japan, North America, and Europe, and causes a serious nuisance problem in several countries. Ali and Majori (1984) reported that 2 saltwater lakes in Orbetello, Central Italy were occupied by Ch. salinarius with over 4,000 larvae/m2. Ali et al. (1985) found that this species prevaled in large numbers (mean larvae density; 5,127/m2) in the saltwater lagoon near Venice Airport, Italy. Seasonal prevalence studies of Ch. salinarius in Nagoya City, Japan in 1995-1997 showed 2 distinct peaks, the first main peak in May-June and the second small one in October-November (Hirvenoja and Michilova, 1998).
In Jinhae City, Korea, Ch. salinarius midges will probably emerge in huge numbers in summer-autumn season of 2006 and the nuisance problem will continue until the harbor construction is completed, unless well-organized control activities are implemented in time.