Identification of novel Leishmania major antigens that elicit IgG2a response in resistant and susceptible mice
Article information
Abstract
Experimental murine models with high, intermediate and low levels of genetically based susceptibility to Leishmania major infection reproduce almost entire spectrum of clinical manifestations of the human disease. There are increasing non-comparative studies on immune responses against isolated antigens of L. major in different murine strains. The aim of the present study was to find out whether there is an antigen that can induce protective immune response in resistant and susceptible murine strains. To do that, crude antigenic extract of procyclic and metacyclic promastigotes of L. major was prepared and subjected to SDS-PAGE electrophoresis. Western-blotting was used to search for antigen(s) capable of raising high antibody level of IgG2a versus IgG1 in the sera of both infected resistant and susceptible strains. Two novel antigens from metacyclic promastigotes of L. major (140 and 152 kDa) were potentially able to induce specific dominant IgG2a responses in BALB/c and C57BL/6 mice. The 2 antigens also reacted with IgG antibody of cutaneous leishmaniasis patients. We confirm that 140 and 152 kDa proteins of L. major promastigotes are inducing IgG production in mice and humans.
INTRODUCTION
The organisms responsible for leishmaniasis are protozoan parasites belong to the genus Leishmania. About 20 species of Leishmania are known to infect man leading to different symptoms ranging from simple self-healing skin ulcers to severe life-threatening disease such as visceral Leishmaniasis, which all together constitute an important public health problem in the endemic area. Experimental murine models with high, low and intermediate levels of genetically based susceptibility to Leishmania infection have reproduced the entire spectrum of clinical manifestations of the human disease with the exception of the mucosal form, and thus provide an excellent system to study the effectiveness of new vaccines (Chang et al., 2003; Gumy et al., 2004).
In experimental murine Leishmaniasis, initiated by subcutaneous injection of the protozoan parasite L. major into mice, 2 extremely different types of disease are observed depending on the strain of mice. In susceptible mice (e.g. BALB/c), the disease is progressive and fatal, while the same parasite causes self-healing with local inflammation in resistant murine strains such as C57BL/6 and CBA/j (Gumy et al., 2004). Infection of BALB/c mice with L. major is commonly used as a model system for cell-mediated immune regulation, since the infection results in a characteristic proliferation of CD4+ T cells exhibiting a Th2 phenotype and a subsequent inability to resolve the disease. Numerous studies demonstrate that BALB/c mice infected with L. major are indeed capable of mounting Th1 responses to distinct Leishmania antigens (Sacks and Anderson, 2004).
It has been shown that the Th1 cytokine, IFN-γ promotes immunoglobulin switching from IgM to the IgG2a isotype. At the same time, IL-4 triggers switch from IgM to IgG1 and IgE. Indeed, IgG2a and IgG1 kinetics indirectly reflect the Th1/Th2 responses. The relative production of these isotypes can thus be used as a marker for the induction of Th1-like and Th2-like immune responses, respectively. In particular, there are several reports introducing vaccines for leishmaniasis in murine model which demonstrate the correlation of Th response and IgG isotypes (Shimizu et al., 2003; Iborra et al., 2004; Tewary et al., 2004; Lange et al., 2004).
In an attempt to identify new immunogenic proteins as a candidate suitable for preparation of cocktail vaccine against leishmaniasis, we utilized western blot analysis with sera from L. major infected BALB/c and C57BL/6 mice and from patients of cutaneous leishmaniasis.
MATERIALS AND METHODS
Parasites
Leishmania major (MRHO/IR/75/ER) was used for the study. The infectivity of the parasites was maintained by regular passages in susceptible BALB/c mice. Parasites were grown in the RPMI medium supplemented with 10% fetal bovine sera, 292 µg/l L-glutamine and 4.5 mg/ml glucose at 27℃. The starting parasite inoculum was 1 × 106/ml. Under these conditions, the stationary phase parasite growth was obtained in 6 days determined by the Peanut agglutination assay (Iborra et al., 2004) and logarithmic phase was obtained in 2 days.
Infection
Four BALB/c and 5 C57BL/6 mice (8 to 9 week-old inbred female mice) subcutaneously (S.C.) received an injection of 2 × 106 stationary phase live promastigotes in 50 µl PBS into their rumps. Sera were obtained from each mouse 10 weeks after infection.
SDS-PAGE
Parasites in stationary or logarithmic phase were harvested from culture, washed 3 times in cold phosphate-buffered saline (PBS) and resuspended at 1 × 109 parasites/ml in 100 mM Tris-HCl, 1 mM EDTA, pH 8 with 1 mM phenylmethylsulfonyl-fluoride (PMSF). Leishmania major lysate was prepared through repeated freezing thawing (4 times) of promastigotes suspended in an equal volume of SDS sample buffer. Leishmania major lysate proteins were separated by SDS-PAGE using 7.5-15% linear gradient polyacrylamide vertical slab gels. Proteins were visualized by staining with 0.025% Coomassie brilliant blue R-250 or silver nitrate and quantified by scanning densitometry.
Western blotting
The reactivity of the IgG class of anti-leishmanial antibodies to promastigote antigens was studied by immunoblotting experiments. The gel-separated proteins were transferred on nitrocellulose membrane. The blotted strips were blocked with 3% (w/v) bovine serum albumin (BSA) solution in PBS containing 0.5% Tween-20 (blocking buffer) and incubated overnight at 4℃ with either murine or human sera (1:50 dilution in blocking buffer). The strips were then washed with immuno-staining buffer (10 mM Tris-HCl, pH 7.4 containing 0.15 M NaCl and 0.3% Tween-20). In the case of murine sera, each strip was incubated with one of the specific monoclonal goat anti-mouse IgG isotypes (IgG1, IgG2a, IgG2b and IgG3) (Sigma, St.Louis, MO, U.S.A.) for 4 hr followed by several washes. The murine strips were incubated with rabbit anti-goat whole IgG-peroxidase conjugate (Sigma). Strips incubated with human sera were incubated with goat antihuman IgG-peroxidase conjugate (Sigma) for 2 hr. Then, all strips were washed 3 times with immunostaining buffer and developed using substrate solution (30 mg diaminobenzidin in 100 ml of 50 mM Tris-HCl and 70 µl H2O2). All the strips were washed by ddH2O and air-dried.
RESULTS
Protein profiles of procyclic and metacyclic L. major promastigotes and scanning densitometry of L. major promastigote proteins revealed different protein expression patterns between procyclic and metacyclic promastigotes. The results showed that some proteins including 152 and 140 kDa, which have not been reported, are metacyclic specific. Amounts of these proteins are very low in comparison with others so that their detection on a silver stained SDS gel is difficult even with high loading quantities (Fig. 1).
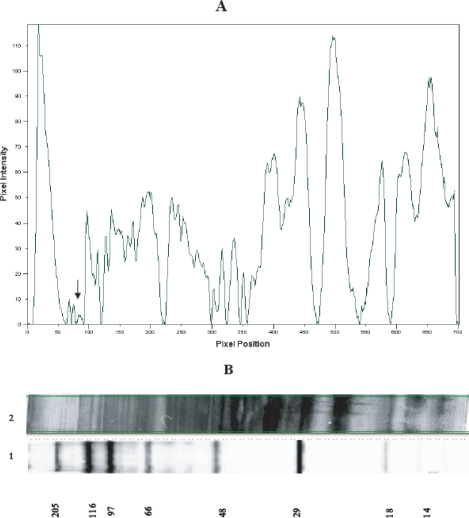
Scanning densitometry diagram of metacyclic L. major proteins (A) which were resolved on a silver stained 7.5-15% gradient SDS-PAGE (B). The band of 152 kDa is indicated by arrow. Lane 1 is wide range molecular weight standard. Lane 2 is metacyclic L. major proteins.
IgG isotype profile of resistant and susceptible murine strains against separated metacyclic L. major promastigote proteins. Fig. 2 shows IgG isotype profile of the sera of 4 susceptible BALB/c mice 10 weeks after infection with L. major. In 2 of them, IgG2a response against 152 and 140 kDa antigenic bands is dominant in comparison with IgG1 and the same results were obtained in all resistant C57BL/6 mice (Fig. 2). We could not detect any visible bands of these molecular weights by immunoblot analysis of procyclic L. major promastigote proteins (data not shown).
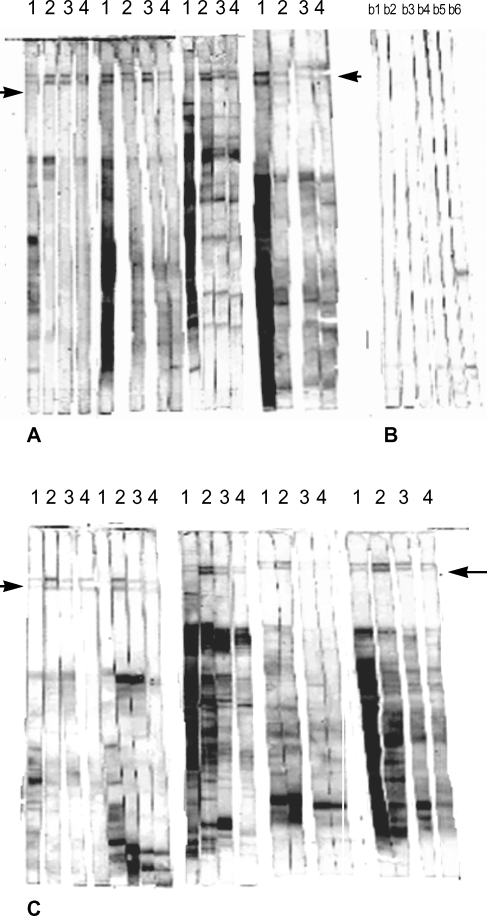
Immunoblot analysis of parasite-specific IgG isotypes (IgG1, IgG2a, IgG2b and IgG3) in L. major infected BALB/c (A), and C57BL/6 mice (C). Control mice from each strain are in B; b1-b3. strips, BALB/c control sera, b4-b6, C57BL/6 control sera. Lanes 1-4 show IgG1, IgG2a, IgG3 and IgG4 reactive bands respectively. Arrows indicate the locations of 152 and 140 kDa antigenic bands.
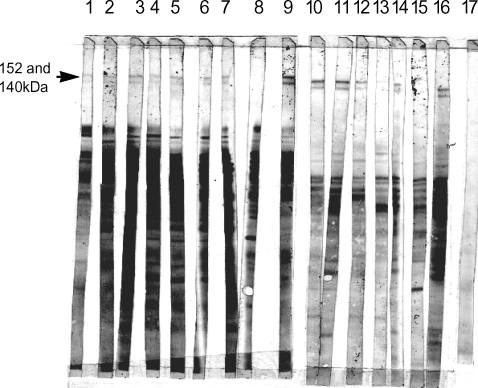
IgG responses of Iranian patients recovered from cutaneous leishmaniasis were observed against separated proteins of L. major metacyclic promastigotes. Further studies with sera of 16 Iranian patients recovered from cutaneous leishmaniasis showed that most of the sera have reactivity against these 2 antigens (Fig. 3).
DISCUSSION
Determining the Th1/Th2 cytokine profile induced by Leishmania antigen and IgG isotype response has been used to identify vaccine candidate(s) in murine models of leishmaniasis. For instance, immunization with LAg-liposome elicited strong antibody responses in mice against L. donovani infection. The titer of IgG1 was significantly higher than IgG2a, IgG2b, and IgG3. Production of IgG1 over IgG2a, IgG2b, and IgG3 suggest a dominance of Th2 response with this liposome-antigen formulation, resulting in weak protection against visceral leishmaniasis (Afrin et al., 2000). Canine antibody IgG, IgG1 and IgG2 anti-FML responses were investigated in dogs vaccinated with the fucose-mannose ligand (FML)-vaccine of L. donovani and in dogs with naturally acquired visceral leishmaniasis. While similar levels of total IgG antibodies were seen in the seropositive naturally infected dogs and in vaccinees, significant differences between the groups were found regarding their IgG1/IgG2 anti-FML antibody compositions. Higher IgG1 absorbancies were seen in infected dogs, while the IgG2 subtype was predominant in pre-immune sera, and in vaccinated animals, both after the first and the third dose. The average ratio between IgG1/IgG2 was then 1.124 for infected animals and 0.733 for FML-vaccinees. Also, a significant increase in IgG2 antibodies was observed from the first to the third vaccine injection. Thus, the analysis of IgG subclasses disclosed a dichotomous response to visceral leishmaniasis; IgG1 associated to natural infection and IgG2 associated to a humoral response subsequent to the FML-vaccine treatment. An IgG1/IgG2 > or = 1 would characterize the sera of visceral leishmaniasis infected animals evolving towards the overt disease while ratios < or = 1 would characterize the sera response of vaccinated protected dogs (de Oliveira et al., 2004).
Qualitative analysis of immunoblot has been studied on Leishmania antigens. Preferential recognition of L. infantum, L. donovani, and L. major antigens by particular subclass of antibodies was demonstrated (Shimizu et al., 2003; Iborra et al., 2004; Tewary et al., 2004; Lange et al., 2004).
BALB/c mice are known as the most susceptible murine strains to L. major infection due to development of Th2 response after infection. In contrast, C57BL/6 mice are known as resistant murine strains against L. major infection due to elevated Th1 responses (Sacks and Anderson, 2004). Nonetheless, IFN-γ producing T cells which recognized LACK antigen was found in L. major infected BALB/c mice (Kelly and Locksley, 2004). In another study, LeIF-specific T cell responses in Leishmania major-infected and uninfected BALB/c mice were characterized. When lymph node cells from infected BALB/c mice were stimulated in vitro with LeIF, only IFN-γ (and no detectable IL-4) was found in the culture supernatant. In addition, LeIF down-regulated Leishmania Ag-specific IL-4 production by lymph node cells from infected BALB/c mice (Skeiky et al., 1998). Therefore, Th1 cells are also found in BALB/c mice with predominant Th2 responses. In addition, there is possibility to induce Th1 response towards the parasite or its antigens. It has been shown that DNA-Salmonella enterica serovar Typhimurium primer-booster vaccination strongly induced Th1 responses against L. major infection in susceptible BALB/c mice. This results in higher IgG2a responses compared to IgG1 responses, higher IFN-γ responses compared to IL-10 CD4+T-cell responses, and enhanced protection (Lange et al., 2004). Mannopentose and dipalmitoylphosphatidylethanolamine (Man5-DPPE)-coated liposome-encapsulated antigens could serve as a vaccine that triggers protection against L. major infection in BALB /c mice (Shimizu et al., 2003).
Based on the above findings we made attempts to find an antigen capable of inducing Th1 responses in both susceptible and resistant mice. In the present study, we found 2 novel antigens (152 and 140 kDa) which were recognized by sera from resistant and susceptible mice, C57BL/6 and BALB/c. Interestingly, the dominant IgG subclass was found to be IgG2a in all sera of C57BL/6 and 2 out of 4 BALB/c mice. Such unique response was not found for other immunoreactive bands. It is unclear to us why 2 BALB/c mice did not present IgG2a dominant response towards these 2 antigens. It is noteworthy that a mixed response of C57BL/6 mice to other antigens was found and the dominant IgG subclass in sera of C57BL/6 mice to some antigens, except the interested antigens, showed a mixture of IgG isotypes. Taken together, our data showed that these 2 antigens per se are able to promote Th1 responses because all C57BL/6 and 50% of BALB/c mice showed dominant IgG2a production.
Intralesional isotype profiles in human localized cutaneous leishmaniasis lesions has been studied and it was shown that the predominant isotypes were IgG1 and IgG2 suggesting a preferential Th1 immune response (Aragort et al, 2001). Antibody response of human cutaneous leishmaniasis cases has been investigated for different antigens such as KMP-11 (Trujillo et al., 1999), CPA and CPB (Rafati et al., 2001) and Histone H1 (Carmelo et al., 2002). In addition, immunoblotting technique has been used to find antigen(s) for differential diagnosis of cutaneous leishmaniasis (Brito et al., 2001; Goncalves et al., 2002). This technique is found a reliable and easy-to-perform for detecting immuno-reactive antigens recognized by sera of patients with history of cutaneous leishmaniasis. In order to see whether human subjects can also recognize these 2 molecules, sera from healthy subjects with previous cutaneous leishmaniasis history were checked using immunoblotting technique and it was found that nearly all subjects recognize these antigens. It would be interesting to know the predominant subclass of IgG. There is no report about any antigen with molecular weight above 130 kDa. This might be due to the very low expression of these antigens.
In conclusion, 2 novel antigens of L. major capable of inducing dominant IgG2a in C57BL/6 and BALB/c mice were introduced. In addition, sera of cured individuals with cutaneous leishmaniasis history were found to be positive for these antigens. Further biochemical and immunological investigations remain to be done.
Notes
This work was financially supported by grants from the Research Council of University of Tehran.