A new method for concentration of proteins in the calcareous corpuscles separated from the spargana of Spirometra erinacei
Article information
Abstract
Calcareous corpuscles are a characteristic structure found in larval and adult stage cestodes. These corpuscles are known to contain several protein components and to possess protein-binding activity. However, the proteins bound to calcareous corpuscles in situ have not been studied. The present study was undertaken to identify the proteins on calcareous corpuscles. Calcareous corpuscles were purified from the plerocercoids (= spargana) of Spirometra erinacei, and serially dissolved using 0.1 M sulfamic acid solution. Collected supernatants were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. The results showed that only the fraction remaining after the 19th dissolved fraction contained proteins. A total of 20 protein molecules were detected in gel, with major bands at 56, 53, 46, 40, 35, 29, 28, 24.5, 21, 19, 16, 13, 10 and 8 kDa. In particular, the proteins corresponding to the 21 and 16 kDa bands were most abundant. Our results demonstrated for the first time the protein contents of the calcareous corpuscles of spargana. Further studies on the functions of these proteins are required.
Calcareous corpuscles are a characteristic structure in cestodes, and are found in the juvenile and adult stages of various tapeworm species (Nieland and von Brand, 1969). Several suggestions have been made on possibilities about their functions, but their exact roles remain unclear. The main components of calcareous corpuscles are inorganic in nature, e.g., calcium and magnesium, and phosphates and carbonates (von Brand et al., 1960). The organic matrix of calcareous corpuscles is composed of carbohydrates, lipids, and relatively few proteins (von Brand and Nylen, 1970). In particular, the proteins are regarded as important components of the structural framework or to act as a substrate for mineral deposition (Nieland and von Brand, 1969). The presence of corpuscular proteins was first demonstrated in worm sections by histochemical staining (Chowdhury et al., 1955; von Brand et al., 1960), and purified corpuscles showed protein binding activities against crude extracts of Platyhelminthes (Yang, 2000). However, no reports are available on the protein profiles of calcareous corpuscles. In the present study, we extracted proteins from the calcareous corpuscles of Spirometra erinacei plerocercoids (sparganum) and determined their molecular profiles by SDS-PAGE.
Spargana were obtained from the east Asian grass snake, Rhabdophis tigrinus, captured on Kanghwa Islands, Kyonggi-do, Korea. Snake tissue debris attached to spargana was removed using a needle, and worms were washed 3 times with sterile phosphate buffered saline (PBS), pH 7.4. To separate calcareous corpuscles, modifications of previously described methods were used. Briefly, spargana were homogenized in a teflon-coated tissue homogenizer, and the homogenized material was centrifuged for 1 hour at 15,000 rpm and 4℃. The pellet so-obtained were composed of two layers, an upper light yellow layer and a lower white layer. The supernatant and upper yellow layers, which contained tissue debris, were removed using a pipette and a syringe. About 10 volumes of sterile PBS were then added and the remaining white pellet was re-suspended. Calcareous corpuscles were separated using Ficoll-Paque plus solution (Amersham Biosciences, Uppsala, Sweden) (Yang, 2000). Spargana debris was observed under a light microscope. Separated corpuscles were collected into a Petri dish and washed 3 times with sterile PBS. These were further confirmed to be corpuscles under a light microscope (Fig. 1A) and were then preserved at -70℃ until required.
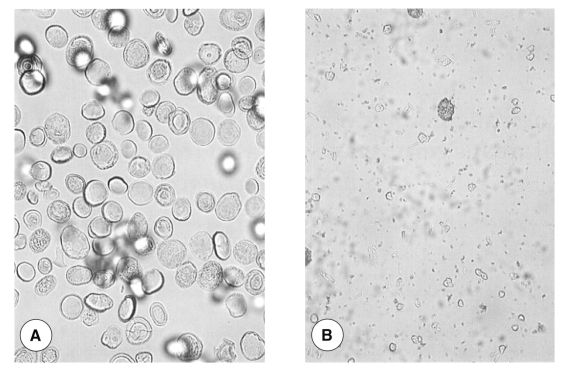
Purified and sulfamic acid-treated calcareous corpuscles of spargana. A: purified calcareous corpuscles from spargana (× 400), B: 0.1 M sulfamic acid-treated calcareous corpuscles (× 400).
We used sulfamic acid to dissolve calcareous corpuscles that had been known as an effective solvent for calcium carbonate precipitate. About 2 volumes of 0.1 M (9.7%) sulfamic acid solution, pH 1.2, were added to corpuscles, which were then dissolved by vortexing vigorously for 1 minute. The solution obtained was then centrifuged for 5 minute at 15,000 rpm and 4℃. The supernatant was collected and residual pellets were retreated with 0.1 M sulfamic acid solution several times. During the course of this process, the corpuscles were observed under a light microscope to confirm dissolution. All supernatants obtained were preserved at -70℃ until required. The presence of proteins in 10-µl samples of supernatants was confirmed by 7.5-15% gradient SDS-PAGE. After electrophoresis, gels were stained using a Silver Staining Kit Protein (Amersham Biosciences).
Fragmented and partially solubilized corpuscles were observed during the dissolution process (Fig. 1B). After 19 times' dissolution, the corpuscles had completely dissolved. Silver stained gel showed that proteins were concentrated in the final (19th) dissolved fraction, whereas relatively small amounts of protein were observed in the first dissolved fraction. Twenty protein molecules were observed at 309, 195, 96, 93.5, 87, 56, 53, 46, 40, 37.5, 35, 29, 28, 24.5, 21, 19, 16, 13, 10 and 8 kDa (Fig. 2). Major proteins were found at 53, 40, 29, 21, 16, 10 and 8 kDa. Two protein molecules, at 21 and 16 kDa, were particularly intense.
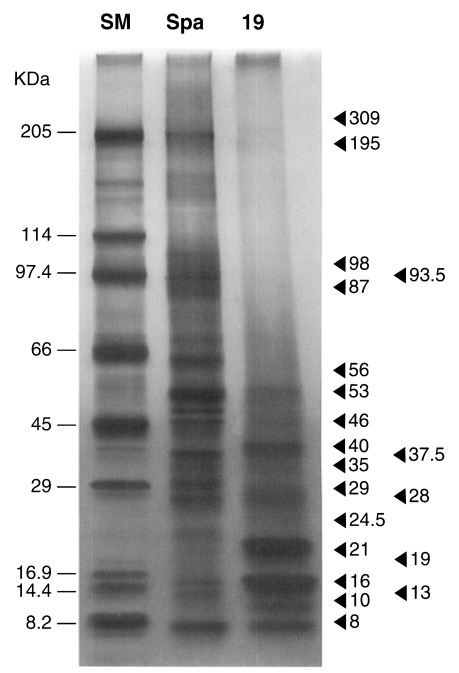
The electrophoretic pattern of the 19th extract of calcareous corpuscles (SM; standard marker, Spa; sparganum antigen, 19; the 19th extract from calcareous corpuscles obtained by repetitive 0.1 M sulfamic acid treatments).
It is known that the calcareous corpuscles of cestodes contain relatively little protein as compared with their inorganic and other organic components (von Brand et al., 1960; von Brand and Nylen, 1970). The amount of proteins in dried corpuscles of Echinococcus granulosus was estimated to be about 4.8-7.8% (von Brand et al., 1965). In the present study, using a serial dissolution process with 0.1 M sulfamic acid, we dissolved calcareous corpuscles and obtained a protein containing solution. Sulfamic acid has been used as an effective solvent for calcium carbonate precipitates (Shepherd and Wyn-Jones, 1996).
In a preliminary study, we found that calcium chelators like ethylene diamine tetraacetic acid (EDTA) and ethylene glycol-β-aminoethylether-N,N,N',N'-tetraacetic acid (EGTA) can also dissolve calcareous corpuscles but that they do not allow the complete separation of protein components (data not shown). It seems that, using sulfamic acid, unbound proteins rebind quickly to remaining fragments of calcareous corpuscles but not in cases using EDTA and EGTA. However, the final fraction obtained using sulfamic acid was not sufficient to allow its protein concentration to be determined. Therefore, the protein concentration of sparganum crude antigen was measured (0.2 mg/ml) to estimate the protein concentration of concentrated extract sample. The protein concentration of the concentrated 19th fraction was estimated to be lower than that of the sparganum crude antigen.
Coomassie blue staining of gels did not allow visualization of the protein bands due to the low protein concentration, and therefore, the more sensitive silver staining was used. It is known that purified calcareous corpuscles of spargana can bind proteins from other species of Platyhelminthes as shown by an artificial protein binding assay (Yang, 2000). Compared to the results of the experimental protein binding assay, protein molecules extracted from calcareous corpuscles in the present study seemed to bind to the corpuscles passively due to their inorganic components.
As shown by SDS-PAGE analysis, the protein profiles of calcareous corpuscles obtained in the present study (Fig. 2) differ considerably from those of Yang (2000) in molecular size and abundance. The proteins observed at 35, 22, 17 and 10 kDa were found to bind to corpuscles (Yang, 2000). In the present study, these seem to correspond to 35, 21, 16, and 10 kDa proteins, respectively. The 21 and 16 kDa proteins were most abundant in corpuscular extracts, whereas the 35 kDa protein was present at a relatively low level. The relationship between calcareous corpuscles and bound proteins is uncertain at the moment. The 10 kDa protein of spargana was found to be distributed in calcareous corpuscles and in subtegumental cells (Chung et al., 2003), and it was suggested that corpuscular proteins should be located in other parts of the worm. In the present study, we separated the proteins of calcareous corpuscles. The results obtained provided clues concerning the biological roles and functions of calcareous corpuscles.
References
Notes
This study was supported by an Inha University Research Grant (INHA-21102-01).