Degranulation of human eosinophils induced by Paragonimus westermani-secreted protease
Article information
Abstract
Eosinophil degranulation is considered to be a key effector function for the killing of helminthic worms and tissue inflammation at worm-infected lesion sites. However, relatively little data are available with regard to eosinophil response after stimulation with worm-secreted products which contain a large quantity of cysteine proteases. In this study, we attempted to determine whether the degranulation of human eosinophils could be induced by the direct stimulation of the excretory-secretory products (ESP) of Paragonimus westermani, which causes pulmonary paragonimiasis in human beings. Incubation of eosinophils for 3 hr with Paragonimus-secreted products resulted in marked degranulation, as evidenced by the release of eosinophil-derived neurotoxin (EDN) in the culture supernatants. Moreover, superoxide anion was produced by eosinophils after stimulation of the ESP. The ESP-induced EDN release was found to be significantly inhibited when the ESP was pretreated with protease inhibitor cocktail or the cysteine protease inhibitor, E-64. These findings suggest that human eosinophils become degranulated in response to P. westermani-secreted proteases, which may contribute to in vivo tissue inflammation around the worms.
Eosinophils play crucial roles in the tissue inflammatory reactions associated with tissue-invasive helminth infections, as well as allergic diseases (Gleich and Adolphson, 1996; Kita et al., 1998). During these inflammatory reactions, eosinophils are stimulated by the appropriate stimuli, which include secretory immunoglobulin A (IgA) (Abu-Ghazaleh et al., 1989), platelet-activating factor (PAF) (Kroegel et al., 1989), complement fragments (e. g., C5a) (Zeck-Kapp et al., 1995), and cytokines (e. g., IL-5, IL-3, and GM-CSF) (Horie et al., 1996). The activated eosinophils release toxic oxygen metabolites and cytotoxic granular proteins, which may contribute to the pathogenesis of eosinophil-mediated tissue inflammation. However, the pathophysiological stimuli involved in the regulation of the eosinophilic effector functions remain largely unknown.
Paragonimus westermani is a tissue-invasive parasite, which causes either pulmonary or extrapulmonary paragonimiasis in humans. The excretory-secretory products (ESP) produced by P. westermani newly excysted metacercariae (PwNEM), which harbor a prodigious amount of cysteine proteases (Chung et al., 1995; Shin and Lee, 2000), play key roles in larval migration in host tissues, and exert modulatory effects on host immune responses (Chung et al., 1995; Chung et al., 1997a, 1997b; Shin and Lee, 2000; Shin et al., 2001). However, the precise roles of the parasite-secreted proteases with regard to the functions of the eosinophils have, thus far, remained enigmatic, although the potential roles of proteases derived from mites (King et al., 1998), fungi (kauffman et al., 2000), and bacteria (Lourbakos et al., 2001a, 2001b) in inflammatory responses have been reported in previous studies. Therefore, we have attempted to investigate the regulatory role of cysteine protease in the ESP secreted by PwNEM in effector functions of human eosinophils.
The ESP was generated from 5,000 PwNEM, as previously described (Shin et al., 2001). The protease activity of the ESP checked by a casein protease assay, using the QuantiCleave™ protease assay kit (Pierce, Rockford, IL, USA). In addition, we measured relative protease activity of the ESP using synthetic dipeptide substrate carbobenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (Cbz-Phe-Arg-AMC) (Sigma, St. Louis, MO, USA) with 2 mM dithioreitol (DTT). One unit of the enzyme activity was determined as the amount of the enzyme that caused releasing of 1 µM of AMC in 1 hr. The enzyme inhibition test was measured with various classes of protease inhibitors such as iodoacetic acid (IAA, 20 µM), trans epoxy-succinly-L-leucyl-amido(4-guanidino) butane (E-64, 10 µM), di-isopropylfluorophosphate (DFP, 2 mM), 4-(amidinophenyl) methanesulphonyl fluoride (APMSF, 10 µM), L-1-chloro-3-[4-tosylamido]-7-amino-2-heptanone · HCl, tosyl lysyl chloromethyl ketone (TLCK 0.1 mM), 1,10-Phenanthroline (2 mM). As shown in Table 1, cysteine protease inhibitors such as IAA and E-64, almost completely inhibited protease activity of the ESP compared to that of non-treated ESP. However, serine protease inhibitors such as DFP and APMSF or a metalloprotease inhibitor (1,10-phenanthroline) did not inhibit the protease activity of the ESP.
Human peripheral blood was collected from normal volunteers without no medication of allergic drugs, and eosinophils were isolated by immunomagnetic negative selection, using anti-human CD16 conjugated with magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described (Shin et al., 2001). The purity of the eosinophils was consistently greater than 95%, as determined by Randolph's staining. Superoxide production was measured by determining the superoxide dismutase-inhibitable reduction of cytochrome c, as reported in a previous work (Shin et al., 2001). In order to quantify the degree of eosinophil degranulation, we measured the concentrations of eosinophil-derived neurotoxin (EDN) in the culture supernatants by specific RIA, as previously described (Shin et al., 2001). In addition, in order to determine the role of the cysteine proteolytic activity of the ESP produced by PwNEM in eosinophil degranulation, the ESP (100 µg/ml) was preincubated with or without a protease inhibitor cocktail (Boeringer Manheim Biochemicals, Germany) or the cysteine protease inhibitor, E-64 (EMD Biosciences, San Diego, CA, USA) at RT for 50 min before incubation with the eosinophils. Data were expressed as mean ± SEM from three independent experiments with different donors. The statistical significance of the differences between the treatment and control groups was assessed by Student's t test. Probability values of less than 0.05 were considered to be significant.
Eosinophils incubated with different concentrations (10, 30, and 100 µg/ml) of ESP exhibited superoxide anion production in a time and concentration-dependent manner (Fig. 1A). After 3 hr of incubation, when the eosinophils were stimulated with 100 µg/ml ESP, 18.1 ± 1.53 nmole of superoxide were produced. This value represents approximately 70% of the superoxide produced by the cells incubated with 1 µM platelet activating factor (PAF). However, little or no production of superoxide was observed when the cells were stimulated with 10 µg/ml ESP or medium alone. Furthermore, as shown in Figure 1B, we found that the ESP strongly induced eosinophil degranulation, as measured by EDN release, in a concentration-dependent manner. At 30 and 100 µg/ml, the ESP induced 170.4 ± 26.3 and 640 ± 122.8 ng release of EDN/106 cells, respectively. The amount of EDN release induced by 100 µg of ESP/ml was 2.3 times as high as that which was induced by 1 µM PAF (280 ± 91.6 ng/106 cells). In contrast, the amounts of EDN release induced by stimulation of 10 µg/ml ESP and medium alone were 58.4 ± 1.4 and 35.2 ± 4.9 ng/106 cells, respectively. These findings suggest that the ESP produced by PwNEM stimulates human eosinophils to induce superoxide production and degranulation. In order to determine whether the ability of the ESP produced by PwNEM to induce eosinophil degranulation might be attributable to the proteolytic activity of cysteine proteases in the ESP, we assessed the inhibitory effects of either a protease inhibitor cocktail or E-64 on the eosinophil degranulation induced by 100 µg of ESP/ml. As shown in Fig. 2, when the ESP was pretreated with the protease inhibitor cocktail, ESP-induced EDN release was significantly inhibited by 47% (P < 0.05) compared to non-treated ESP. Similarly, pretreatment with E-64 also resulted in the significant inhibition of ESP-induced eosinophil degranulation by 46% (P < 0.05). By contrast, PAF-induced eosinophil degranulation was not found to be affected by pretreatment of the PAF with protease inhibitor cocktail (mean inhibition, 1.6%). These results suggest that cysteine proteolytic activity plays a major role in the activation of eosinophils, and functions as a response to the ESP which is produced by the PwNEM. Although we were unable to exclude the possibility that other components in the ESP might be involved in the degranulation of the eosinophils, our finding that the regulation of eosinophil's effector functions induced by the ESP produced by PwNEN might constitute an important clue toward our understanding of the pathophysiological roles of worm-secreted cysteine proteases with regard to eosinophilic inflammation at worm-infected lesion sites (Racz et al., 1982). Recently, the proteolytic activity of cysteine or serine protease derived from oral pathogens and mite allergens has been demonstrated to exert regulatory effects on the behavior of human cells through a family of G protein-coupled protease-activated receptors (PARs) (Lourbakos et al., 2001a, 2001b; Sun et al., 2001; Asokananthan et al., 2002). The cleavage of the extracellular NH2-domains of these receptors via the proteolytic actions of extracellular protease exposes a specific new-NH2 terminus, which is composed of six or more amino acids, which act as tethered ligands, and activate the seven-transmembrane domains of the cleaved receptor (Dery et al., 1998; Macfarlane, 2001). Thus, cells bearing PARs detect different extracellular protease concentrations according to the rate at which receptor cleavage proceeds (Ishii et al., 1993). Moreover, human eosinophils can be activated by serine protease trypsin or cysteine proteases such as papain via PAR2, and thereby induce the production of the superoxide anion, as well as eosinophilic degranulation (Miike et al., 2001; Miike and Kita, 2003). Thus, it has been hypothesized that the eosinophil's responses, as induced by Paragonimus-secreted cysteine protease, may be transmitted through PAR-driven mechanisms. This warrants further inquiry into the signaling interactions occurring between the worm-secreted cysteine protease and the PARs in eosinophils.
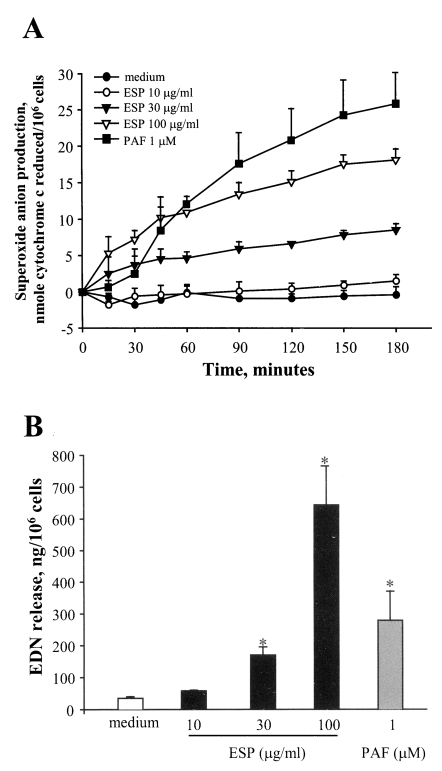
Superoxide anion production (A) and degranulation (B) by human eosinophils stimulated with ESP produced by PwNEM. Eosinophils were stimulated with three different concentrations (10, 30 and 100 µg/ml) of ESP, PAF (1 µM), or medium alone, for up to 3 hr at 37℃. Superoxide production was then measured by determining the superoxide dismutase-inhibitable reduction of cytochrome c. After 3 hr of incubation, the concentrations of EDN in the cell-free supernatants were measured by RIA, as an index of eosinophil degranulation. Data are expressed as means ± SEM from three independent experiments with different eosinophil donors. *, denotes significant differences (P < 0.05) as compared with the medium alone.

Effects of protease inhibitor (PI) cocktail or cysteine protease inhibitor (E-64) on the ESP-induced eosinophil degranulation. The ESP by PwNEM (100 µg/ml) or PAF (1 µM) were preincubated with or without protease inhibitor cocktail solution (3 µl) or E-64 (final conc., 10 µM) for 50 min at RT, and eosinophils were stimulated with the pretreated samples for 3 hr at 37℃. Degranulation was measured as described in Fig. 1. The data were normalized to the values of the stimulus alone, without protease inhibitor cocktail or E-64. This value was considered 100%. Protease inhibitor cocktail or E-64 at the indicated concentrations in this experiment did not show any degranulative effect on eosinophils. Data are presented as means ± SEM of three independent experiments with different eosinophil donors. *, denotes significant differences (P < 0.05) compared with ESP without protease inhibitor cocktail or E-64.
References
Notes
This study was supported by a grant (#HMP-02-PJ1-PG3-21299-0001) of the grant 2002 Good Health R&D Project, Ministry of Health & Welfare, Korea.
