Abstract
After collecting calcareous corpuscles from plerocercoid of Spirometra mansoni (sparganum), we evaluated the antigenic values of calcareous corpuscles binding proteins obtained from the cyst fluid of Taenia solium metacestodes. Immunoblot analysis revealed that cysticercosis patient sera strongly recognized 10 and 95 kDa calcareous corpuscles binding proteins. This result demonstrated that calcareous corpuscles are bound with major secretory antigenic proteins, which is possibly involved in the secretory pathways of the 10 and 95 kDa proteins presenting in the cyst fluid of T. solium metacestodes.
-
Key words: sparganum, calcareous corpuscles binding protein, antigen, Taenia solium metacestodes
Calcareous corpuscles, specific organelles found in Platyhelminthes, may have many biologic functions. Although several studies have been published on the formation and compositions of calcareous corpuscles (
von Brand et al., 1960,
1965,
1969), their exact roles in parasites are poorly understood. Cysticercosis is a worldwide reported disease caused by the systemic spreading of
Taenia solium metacestodes. A 10 kDa protein is highly specific among the several antigenic molecules when diagnosing cysticercosis by immunoblotting, in cyst fluid of
T. solium metacestodes. We published in a previous study that 10, 17, 22 and 95 kDa are calcareous corpuscles binding proteins in the cyst fluid of
T. solium metacestodes (
Yang, 2000). In the present study, calcareous corpuscles binding proteins in the cyst fluid of
T. solium metacestodes, including the 10 and 95 kDa proteins, were evaluated as to whether they react with cysticercosis patient sera.
Calcareous corpuscles of the sparganum were separated as described by Yang (
2000) using Ficoll-Paque plus (Amersham Pharmacia Biotech, USA) and stored in 5 volumes of distilled water at 4℃ until required. After collecting calcareous corpuscles, 50 µg/ml of cyst fluid of
T. solium metacestodes was reacted with calcareous corpuscles at 4℃ overnight. After binding with this cyst fluid of
T. solium metacestodes, calcareous corpuscles binding proteins were separated by 7.5-15% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF; Millipore, Bedford, MA, USA) membranes. Membrane strips were then reacted with cysticercosis patient sera and parasite infected patients sera at a 1:100 dilution in casein buffer (0.5% casein, 20 mM Tris, 150 mM NaCl), washed with PBS/T (0.5% Tween-20). The membranes were then reacted with peroxidase conjugated antihuman IgG antibody (Fc specific; Cappel, Westchester, PA, USA) diluted 1: 2,000 in casein buffer. Colors were developed with 4- chloro-1-naphthol (Sigma, St. Louis, MO, USA).
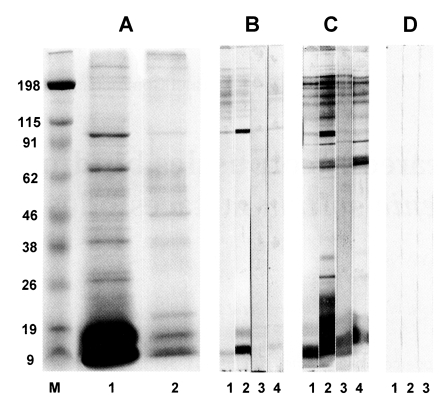
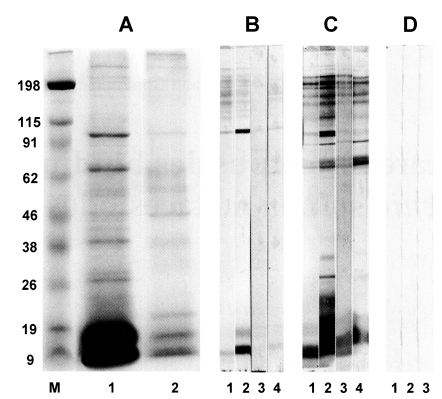
Calcareous corpuscles were found to bind with 10, 17, 22, 35 and 95 kDa molecules in the cyst fluid of
T. solium metacestodes (
Fig. 1A). The bound 10 and 95kDa calcareous corpuscles binding proteins also commonly react with cysticercosis patient sera on immunoblots (
Fig. 1B). Antigenic protein of 10 kDa is known to be a subunit of a 150 kDa protein in the cyst fluid of
T. solium metacestodes and is a highly specific and sensitive antigen for the diagnosis of cysticercosis (
Cho et al., 1988,
1992;
Yang et al., 1998). This molecule has been cloned and its antigenic sites analyzed (
Chung et al., 1999,
2002). The 10 kDa recombinant molecule (CyDA) was found to be reactive in 97% of active neurocysticercosis patients and showed specificity of 98% (
Chung et al., 1999). Although we have not confirmed the results of the calcareous corpuscles binding experiment with the recombinant protein, we found that the calcareous corpuscles binding protein, 10 kDa, in the cyst fluid of
T. solium metacestodes react with cysticercosis patient sera by immunoblotting. Another calcareous corpuscle binding protein, 95 kDa, showed a variable reaction pattern in terms of its intensity. This 95 kDa molecule cross reacts with other helminthic infection sera (
Olivo et al., 1988;
Yang et al., 1998). Calcareous corpuscles bound proteins including the 95 kDa protein are N-glycosidase F sensitive (complex type N-linked oligosaccharide specific) showing molecular shifts on SDS-PAGE (data not shown). This finding concord with the result of strong glycoprotein reactions in the lamellae of calcareous corpuscles in
Trilocularia acanthiaevulgaris (
McCullough and Fairweather, 1987). In this regard, antigenic glycoproteins in the cyst fluid of
T. solium metacestodes are usually secretory proteins, and calcareous corpuscles may be involved in the secretory pathways of glycoproteins. In this paper, we demonstrated the antigenic activity of calcareous corpuscles binding proteins in the cyst fluid of
T. solium metacestodes using cysticercosis patient sera. More studies are required to explain the molecular and biochemical properties of the 10 and 95 kDa calcareous corpuscles binding proteins.
References
- 1. Cho SY, Kim SI, Kang SY, Kong Y. Biochemical properties of a purified protein in cystic fluid of Taenia solium metacestodes. Korean J Parasitol 1988;26:87-94.
- 2. Cho SY, Kong Y, Kim SI, Kang SY. Measurement of150 kDa protein of Taenia solium metacestodes by antibody-sandwich ELISA in cerebrospinal fluid of neurocysticercosis patients. Korean J Parasitol 1992;30:299-307.
- 3. Chung JY, Bahk YY, Huh S, Kang SY, Kong Y, Cho SY. A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J Infect Dis 1999;180:1307-1315.
- 4. Chung JY, Yun DH, Eom KS, Kang SY, Kong Y, Cho SY. Taenia solium: identification of specific antibody binding regions of metacestode 10-kDa protein. Exp Parasitol 2002;100:87-94.
- 5. McCullough JS, Fairweather I. The structure, composition, formation and possible functions of calcareous corpuscles in Trilocularia acanthiaevulgaris Olsson (Cestoda, Tetraphyllidea). Parasitol Res 1987;74:175-182.
- 6. Olivo A, Plancarte A, Flisser A. Presence of antigen B from Taenia solium cysticercus in other platyhelminthes. Int J Parasitol 1988;18:543-545.
- 7. von Brand T, Mercado TI, Nylen MU, Scott DB. Observation on function, composition, and structure of cestode calcareous corpuscles. Exp Parasitol 1960;9:205-214.
- 8. von Brand T, Nylen MU, Martin GN, Churchwell FK, Stites E. Cestode calcareous corpuscles: phosphate relationships, crystallization patterns and variations in size and shape. Exp Parasitol 1969;25:291-310.
- 9. von Brand T, Scott DB, Nylen MU, Pugh MH. Variation in the mineralogical composition of cestode calcareous corpuscles. Exp Parasitol 1965;16:382-391.
- 10. Yang HJ. Separation of calcareous corpuscles from plerocercoids of Spirometra mansoni and their binding proteins. Parasitol Res 2000;86:781-782.
- 11. Yang HJ, Chung JY, Yun D, et al. Immunoblot analysis of a 10 kDa antigen in cyst fluid of Taenia solium metacestodes. Parasite Immunol 1998;20:483-488.
Fig. 17.5-15% SDS-PAGE and immunoblot analysis of calcareous corpuscles binding proteins in the cyst fluid of Taenia solium metacestodes. (A) Cyst fluid of T. solium metacestodes (lane 1), calcareous corpuscles binding proteins in the cyst fluid of T. solium metacestodes (lane 2). (B) Calcareous corpuscles binding proteins reacting with cysticercosis patient sera (lane 1-4). (C) cyst fluid of T. solium metacestodes reacting with cysticercosis patient sera (lane 1-4). Number represents individual patients, respectively. (D) Calcareous corpuscles binding proteins reacting with serum pool of sparganosis (lane 1), clonorchiasis (lane 2) and toxoplasmosis (lane 3) patients sera. Note that the 10 kDa and 95 kDa proteins showing a strong antigenicity in both calcareous corpuscles binding proteins and the cyst fluid of T. solium metacestodes. M, standard marker.
Citations
Citations to this article as recorded by

- Fasciclin-calcareous corpuscle binary complex mediated protein-protein interactions in Taenia solium metacestode
Chun-Seob Ahn, Jeong-Geun Kim, Young-An Bae, Seon-Hee Kim, Joo-Ho Shin, Yichao Yang, Insug Kang, Yoon Kong
Parasites & Vectors.2017;[Epub] CrossRef - Morphometric characteristics of the metacestode Echinococcus vogeli Rausch & Bernstein, 1972 in human infections from the northern region of Brazil
F. Almeida, F. Oliveira, R. Neves, N. Siqueira, R. Rodrigues-Silva, D. Daipert-Garcia, J.R. Machado-Silva
Journal of Helminthology.2015; 89(4): 480. CrossRef - Identification and functional characterization of alpha-enolase from Taenia pisiformis metacestode
Shaohua Zhang, Aijiang Guo, Xueliang Zhu, Yanan You, Junling Hou, Qiuxia Wang, Xuenong Luo, Xuepeng Cai
Acta Tropica.2015; 144: 31. CrossRef - In vitro excystation of Echinostoma paraensei (Digenea: Echinostomatidae) metacercariae assessed by light microscopy, morphometry and confocal laser scanning microscopy
Joyce Souza, Juberlan Garcia, Renata H. Neves, José Roberto Machado-Silva, Arnaldo Maldonado
Experimental Parasitology.2013; 135(4): 701. CrossRef