Abstract
The 150 kDa protein of cyst fluid (CF) of Taenia solium metacestodes was purified by ammonium sulfate fractionation and Superose 6 HR gel filtration chromatography. The purified protein consisted of three subunits (15, 10 and 7 kDa proteins), which were analyzed with the use of a 7.5-15% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Immunofluorescence study was carried out by using immunize specific polyclonal antibody. Positive reactions were noticed at bladder walls, calcareous corpuscles, granules of cyst fluid and some host tissue surrounding the bladder wall of the metacestodes. These results suggest that the 150 kDa protein was secreted into host tissues, inducing immune responses in the host, and it may play important roles in the cellular physiology of the parasites.
-
Key words: Taenia solium metacestode, cyst fluid, immunofluorescence
Diagnoses of cysticercosis are often complex procedures based on clinical and, radiological findings, and which the aid of immunological and epidemiological data (
Del Brutto et al., 2001). The diagnostic antigens of cysticercosis have been well illustrated in several papers (
Cho et al., 1988;
Tsang et al., 1989;
Yang et al.,1998;
Chung et al., 1999,
2002). The promising diagnostic antigen, 10 kDa, from CF of
T. solium metacestodes, is highly specific for neurocysticercosis (
Yang et al., 1998). Recombinant 10 kDa antigenic protein has been proven useful for the diagnosis of active neurocysticercosis with an efficacy of 90% (
Chung et al., 1999). The 10 kDa antigen is one of the subunits consisting a 150 kDa protein in the CF of
T. solium metacestodes (
Cho et al., 1988). Purified 150 kDa protein consists of three subunits (15, 10 and 7 kDa proteins), and immunoblot studies have shown that strong reactions to these polypeptides are highly specific for cysticercosis (
Cho et al., 1988). Although the protein has been regarded as a plausible diagnostic antigen, it is not yet known where the protein is located, and the function of this protein remains unclear. In this study, the 150 kDa protein, a major constituent protein of CF, was purified by column chromatography and localized in
T. solium metacestodes using a specific polyclonal antibody.
The CF of
T. solium metacestodes was prepared from naturally infected pigs, as was described in a previous study (
Yang et al., 1998). For the purification of 150 kDa, purification procedures were carried out using an ammonium sulfate precipitation followed by gel filtration equipped with AKTA-FPLC system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) (
Chung et al., 2002). Briefly, CF crude extracts (10 ml) was saturated with ammonium sulfate up to 80%, the precipitated proteins were discarded by centrifugation and the resulting supernatant was collected. After dialysis and concentration, the supernatant was loaded onto a Superose 6 HR 10/30 column (Amersham Pharmacia Biotech, Piscataway, NJ, USA) that was previously equilibrated with 50 mM Tris-HCl buffer (pH 7.5) containing 0.15 M NaCl. The column was washed with the above buffer and the fractions of 150 kDa were detected by SDS-PAGE. The 150 kDa fractions were collected and concentrated for further study.
BALB/c mice were used to produce the polyclonal antibody of the purified protein by the methods of a previous study (
Chung et al., 2002). For immunohistochemical study, the metacestodes in pork muscle were fixed in 10% neutral formalin, embedded in paraffin, and cut into ribbon with a 6 µm thickness. The paraffin ribbons of
T. solium metacestode were incubated in phosphate buffered saline (PBS) for 2 hr at room temperature with 1:100 diluted mouse immune sera sensitized against the purified protein. As the negative control, the pre-immune BALB/c mouse serum was incubated in PBS with 1: 100 diluted for 2 hr. The ribbons were washed twice with PBS and incubated for 1 hr with fluorescein isocyanate (FITC)-labeled antimouse IgG antibody (Jackson immunoresearch laboratory, PA, USA) diluted 1:50, and then, the ribbons were washed twice with PBS. After observation with a fluorescence microscope (Olympus AX 70, Japan), the ribbons were washed thoroughly with PBS, and then, stained with hematoxylin to compare the organs that reacted with the immune sera.
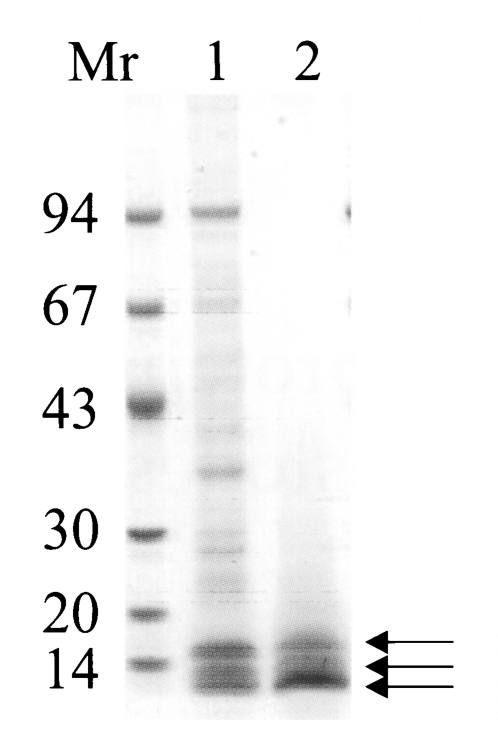
The purified 150 kDa protein, a major constituent molecule in CF crude extracts, migrated at molecular weights of 15, 10 and 7 kDa on a 7.5-15% gradient SDS-PAGE (
Fig. 1), and this procedure correlated with the results of a previous study (
Cho et al., 1988). The native molecular weight of the protein was estimated to be about 150 kDa by a Superose 6 HR 10/30 gel filtration, and the purified protein was soluble even at high concentration of ammonium sulfate solution (up to 80%).
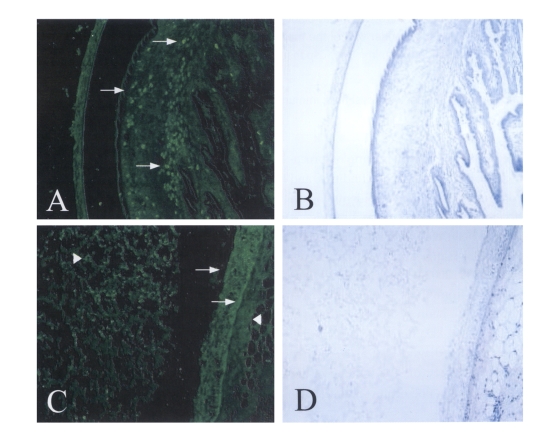
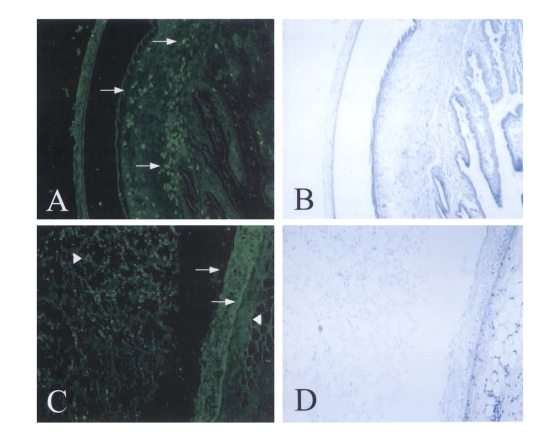
Immunofluorecence staining on
T. solium metacestodes sections using polyclonal immunized serum clearly revealed that the 150 kDa protein was mainly distributed at the bladder walls and calcareous corpuscles of the spiral canal of
T. solium metacestodes (
Fig. 2 A&C). The positive reactions were also observed at the granules of the CF and at host tissues surrounding the bladder walls of
T. solium metacestodes. The inner and outer walls of the bladder reacted equivalently to the polyclonal antibody, although all the calcareous corpuscles of the scolex did not react positively (
Fig. 2 A&C). Another secreted diagnostic antigen, so called 'Antigen B', was distributed at tegumentary cytons of the bladder wall and in the lumen of the spiral canal of the metacestode scolex (
Laclette et al., 1987). The purified 150 kDa protein was secreted secreted into host tissues surrounding the parasite and it induced immune responses. Also, secreted 150 kDa protein in the CF of
T. solium metacestodes may have secretory pathways with the calcareous corpuscles, which may have various cellular physiological processes by regulating mineral components in cestodes (
Pawlowski et al., 1988). Its relationship with the calcareous corpuscles should be investigated in further studies.
Notes
-
This work was supported by Korea Research Foundation Grant(KRF-2001-003-F00035).
ACKNOWLEDGMENTS
Authors deeply thank Professors Seung-Yull Cho and Shin-Yong Kang for providing some materials and also valuable guidance in research of cysticercosis.
References
- 1. Cho SY, Kim SI, Kang SY, Kong Y. Biochemical properties of a purified protein of Taenia solium metacestodes. Korean J Parasitol 1988;26:87-94.
- 2. Chung JY, Bahk YY, Huh S, Kang SY, Kong Y, Cho SY. A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J Infect Dis 1999;180:1307-1315.
- 3. Chung JY, Yun DH, Eom KS, Kang SY, Kong Y, Cho SY. Taenia solium: identification of specific antibody binding regions of metacestode 10-kDa protein. Exp Parasitol 2002;100:87-94.
- 4. Chung YB, Lee M, Yang HJ, et al. Characterization of partially purified 8 kDa antigenic protein of Clonorchis sinensis. Korean J Parasitol 2002;40:83-88.
- 5. Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001;57:177-183.
- 6. Laclette JP, Merchant MT, Wilms K. Histological and ultrastructural localization of antigen B in the metacestode of Taenia solium. J Parasitol 1987;73:121-129.
- 7. Pawlowski ID, Yap KW, Thompson RC. Observation on the possible origin, formation and structure of calcareous corpuscles in taeniid cestodes. Parasitol Res 1988;74:293-296.
- 8. Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 1989;159:50-59.
- 9. Yang HJ, Chung JY, Yun D, et al. Immunoblot analysis of a 10 kDa antigen in cyst fluid of Taenia solium metacestodes. Parasite Immunol 1998;20:483-488.
Fig. 1Purified the 150 kDa protein was analyzed on 7.5-15% gradient SDS-PAGE. Lane 1, crude extracts of CF; lane 2, purified protein. Arrows indicate that the purified 150 kDa protein was separated into three major subunits (Mr, standard marker proteins).
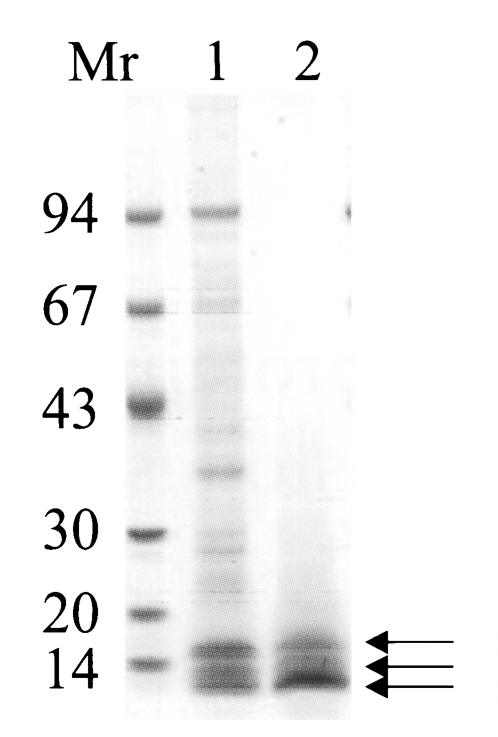
Fig. 2Immunofluorescence staining of 150 kDa protein with specific immune serum.A & C. Positive reactions were distributed at the calcareous corpuscle, bladder walls of the metacestode (arrows) as well as the granules of CF, host tissues surrounding the bladder wall of the metacestode (arrowheads).B & D. The same slides of hematoxylin-eosin staining after observation with fluorescence microscope and washing with PBS buffer (× 100).
Citations
Citations to this article as recorded by

- Taenia saginata: Production and characterization of monoclonal antibodies against Taenia saginata metacestode antigens
Josy Campanhã Vicentini-Oliveira, Marjorie A. Golim, Silvana de Cássia Paulan, Germano Francisco Biondi, Rosana Rossi-Ferreira, Elenice Deffune, Cáris Maroni Nunes
Experimental Parasitology.2010; 126(4): 621. CrossRef