Development of resistance to reinfection by Clonorchis sinensis in rats
Article information
Abstract
We investigated the induction of resistance to Clonorchis sinensis infection by prior infection in rat and hamster models. Animals were challenged with C. sinensis metacercariae, then treated with praziquantel and reinfected. Worm recovery rate in reinfected animals was used to estimate resistance to reinfection. The determined resistance rates to reinfection in rats and hamsters were 97.7% and 10.3%, respectively. In rats, cure from the primary infection of C. sinensis increased resistant to reinfection, and the greatert the worm burden and the longer the duration of primary infection, the higher was the resistance rate. For primary infection doses of 10, 40 and 100 metacercariae per rat, the resistance rates were 87.4%, 93.8% and 98.4%, respectively. The resistance rates in rats after 2 or 8-week primary infection were 78.7% and 95.3%, respectively. All worms recovered from reinfected rats were immature. When cured rats were administered with methylprednisolone, resistance to reinfection became impaired. These findings indicate that rats develop a high degree of resistance to reinfection by C. sinensis after cure. The growths and maturations of reinfected worms were also impaired.
INTRODUCTION
The Chinese liver fluke, Clonorchis sinensis, is widely distributed in Korea, China, East Russia and Vietnam, in regions where residents eat raw or undercooked freshwater fish carrying C. sinensis (Crompton, 1999). The adult fluke inhabits the biliary passages of humans and mammals, and provokes epithelial hyperplasia of the biliary mucosa and periductal fibrosis. Frequent symptoms of human clonorchiasis are a dull epigastric fullness or pain, mild fever, loss of appetite, diarrhea, and jaundice. Moreover, clonorchiasis has been proven epidemiologically and experimentally to be associated with cholangiocarcinoma (Rim, 1986; Chen et al., 1994; Lee et al., 1997; Watanapa and Watanapa, 2002).
Epidemiological studies suggest that humans do not develop any resistance to reinfection or superinfection by C. sinensis, and that reinfection readily occurs upon re-exposure throughout life in those accustomed to consuming undercooked fish in endemic areas (Seo et al., 1981; Hong et al., 1994). However, persons in schistosomiasis endemic areas have been shown to express different levels of resistance to reinfection (Hagan et al., 1991; Caldas et al., 2000). In addition, hamsters have been reported to be resistant to reinfection by Opisthorchis viverrini (Flavell, 1982) and rats were found to be resistant to reinfection by Fasciola hepatica or Schistosoma mansoni. Moreover, in the rat the mechanism of this resistance was found to be related to the development of acquired immunity in the host (Doy et al., 1978; Cetre et al., 1999). Protective immunity has been suggested in rats reinfected with C. sinensis (Quan et al., 2000), however, there is no clear evidence of resistance to reinfection by C. sinensis so far. Therefore, we undertook this study to observe whether exposure to clonorchiasis develops resistance to reinfection in experimental animals. Resistance was further evaluated according to the intensity and duration of the primary infection in rats, and the potential effect of this developed resistance was examined to determine its consequences on worm development in reinfected rats. Rats were also immunosuppressed to determine whether immunity plays a role in resistance to C. sinensis reinfection.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (4 weeks old) and female Syrian golden hamsters (5-6 weeks old) were divided into different groups according to the study infection protocol (Fig. 1). The animals were kept under barrier conditions at the Seoul National University College of Medicine and fed a commercial pelleted diet and piped water. All of the procedures for animal care and experiment followed the guidelines and recommendations of the university.
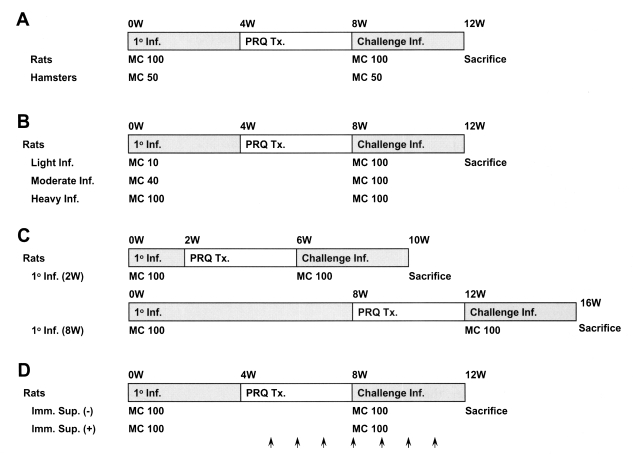
The scheme of infection for observation of resistance to reinfection by Clonorchis sinensis in animals. A, resistance to reinfection in rats and hamsters; B, resistance to reinfection in rats at three different metacercariae doses; light (10/animal), moderate (40/animal), and heavy (100/animal); C, reinfection resistance in rats according to the duration of the primary infection; D, the effect of immunosuppression on resistance to reinfection in rats. Methylprednisolone acetate (Depo-medrol®, arrows), 10 mg/kg, was injected hypodermally once a week for 3 weeks before the reinfection until 4 weeks post-reinfection. 1° Inf., primary infection; Challenge Inf., challenge infection; MC, metacercariae; PZQ TX., praziquantel treatment.
Preparation of C. sinensis metacercariae
The freshwater fish, Pseudorasbora parva collected in Shenyang, China, were digested in acid pepsin solution, and C. sinensis metacercariae were isolated as described previously by Chung et al. (2002). The freshly isolated metacercariae were introduced into the stomach through a gavage needle.
Observation of resistance to C. sinensis reinfection in experimental animals
Fig. 1 shows the scheme of the infection for observation of resistance to reinfection in experimental animals. Rats or hamsters were orally infected with 100 or 50 metacercariae, and treated with praziquantel (Distocide®, Shinpoong Pharmaceutical Co., Seoul, Korea), 100 mg/kg for rats or 50 mg/kg for hamsters, for 3 days, 4 weeks after the primary infection. The effect of treatment was confirmed by stool egg negativity 4 weeks after treatment; cured animals were challenged with the same number of metacercariae as used in their initial challenge. Animals were sacrificed by deep anesthesia 4 weeks after the primary infection or after reinfection, and C. sinensis worms were recovered from the liver and bile duct under a stereomicroscope. Two kinds of controls, primary infection and reinfection controls, were used to confirm that the metacercariae used for the primary infection or the reinfection retained their infectivity. The resistance rate against reinfection was calculated using the formula:
Resistance rate (%) = ((C-R)/C) × 100
Resistance to reinfection in rats was also observed in relation to different intensities of primary infection, i.e., light (10 metacercariae per rat), moderate (40) or heavy (100), and with different durations, short (2 weeks) or long (8 weeks) (Fig. 1). Resistance to reinfection was further compared between normal and immunosuppressed rats, which received 10 mg/kg methylprednisolone acetate (Depo-medrol®, Pharmacia Korea, Hwaseong, Gyonggi, Korea) hypodermally once a week for 3 weeks before the reinfection until 4 weeks post-reinfection (Fig. 1).
Effects of reinfection resistance on worm development
Worms were recovered from the liver and bile ducts of rats. Fifteen worms recovered from each animal were fixed with 10% formalin under a cover glass and stained with acetocarmine to evaluate the effect of resistance on worm development. The worms were classified as 'juvenile' or 'mature' based on the presence of eggs in the uterus, and the sizes of the body and suckers were also measured.
Statistical analysis
The statistical significance of group mean worm recovery and resistance rates was determined by oneway ANOVA followed by the Tukey test. Data were analyzed by SPSS for Windows version 10.0 (SPSS Inc., Chicago, IL).
RESULTS
Resistance to reinfection in experimental animals
The worm recovery rates of the primary and the unchallenged reinfection control groups were not significantly different (p = 0.440, Table 1), showing that the infectivities of the metacercariae used for the primary infection and for the reinfection had not altered. The worm recovery rate in reinfected rats was significantly reduced at 1.4% versus the 62% shown by the reinfection control (p < 0.001), demonstrating that the rats developed a high level of resistance to C. sinensis reinfection (Table 1). However, the worm recovery rates in hamsters were not significantly different between in the reinfection control and reinfected animals (p = 0.526), showing that hamsters are equally susceptible to primary infection and reinfection by C. sinensis.
Resistance to reinfection by primary infection intensity in rats
We also investigated the resistance patterns in reinfected rats with respect to the intensity of the primary infection. The worm recovery rate after reinfection was significantly reduced even in lightly infected rats (p < 0.001) (Fig. 2), and reduced in a dose-dependent manner with the primary infection intensity, showing that resistance rates in rats increased linearly with the infection dose (Fig. 2). The resistance rates of the lightly and heavily infected groups were significantly different (p = 0.027).
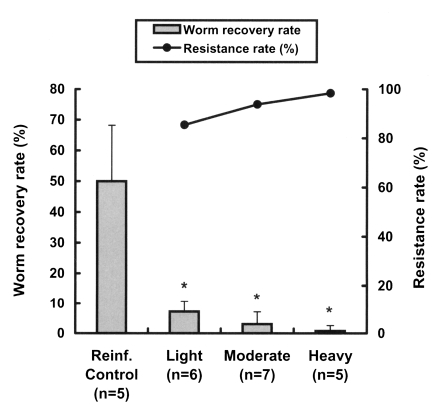
Worm recovery and resistance rates to reinfection with Clonorchis sinensis at different primary infection intensities in rats. Rats were infected with 10 (light), 40 (moderate) and 100 (heavy) metacercariae, respectively, then treated with 100 mg/kg praziquantel 4 weeks after the primary infection, and finally challenged with 100 metacercariae 4 weeks after the praziquantel treatment. Resistance rates were estimated from worm recovery rates in reinfected animals versus non-previously infected controls. Asterisks (*) denote significantly different from the control group (p < 0.001).
Resistance to reinfection by duration of primary infection in rats
Rats, which were primarily infected for two different durations, short (2 weeks) and long (8 weeks), developed resistance to reinfection by C. sinensis (Fig. 3. The worm recovery and resistance rates in rats of 2-week group were 11.8% and 78.7%, while those of rats in the 8-week group were 2.6% and 95.3%, respectively. This difference between the two groups was statistically significant (p < 0.001), and thus, a longer primary infection was found to be associated with a higher resistance rate.
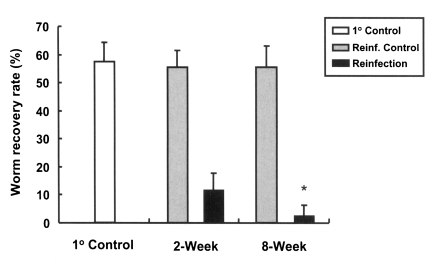
Worm recovery rates in reinfected rats for primary infections of different duration. An asterisk (*) denotes significantly different from rats with a 2-week duration primary infection (p < 0.001). 1° Control, primary infection control; Reinf. Control, reinfection control; Reinfection, reinfected rats.
Effect of immunosuppression on resistance to reinfection in rats and mice
Rats were immunosuppressed to determine whether immunity plays a role in the development of resistance to C. sinensis reinfection. Immunosuppressed rats showed a worm recovery rate of 50.5%, which was similar to the 54.8% of the control group and significantly higher than the 6.8% of immunocompetent reinfected rats (p < 0.001, Fig. 4), showing that resistance in reinfected rats is significantly impaired by immunosuppression (p < 0.001).
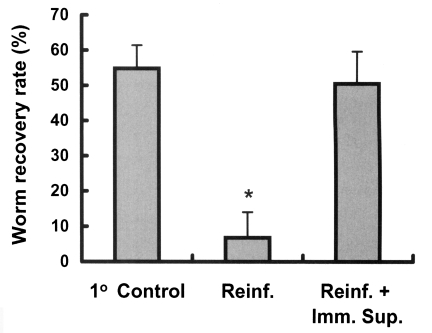
The effect of immunosuppression on the worm recovery rate in reinfected rats. Animals were immunosuppressed using hypodermal injections of methylprednisolone acetate (Depo-medrol®), 10 mg/kg, weekly from 3 weeks before reinfection until 4 weeks after reinfection. An asterisk (*) denotes significantly different from the primary control and the immunosuppressed reinfected group (p < 0.001). 1° Control, primary infection control; Reinf., reinfection; Reinf.+Imm.Sup., reinfection with immunosuppression.
Morphological observations of worms from immunocompetent and immunosuppressed rats
When the sizes of the bodies, and of the oral and ventral suckers of recovered worms were compared, flukes from immunosuppressed, reinfected rats were found to be larger than those of the other two groups (p < 0.001, Table 5), and worms from primary infected rats were significantly larger than those from immunocompetent, reinfected rats (p < 0.001). All worms recovered from immunosuppressed reinfected rats were more mature and larger than those from primary infected rats (p < 0.001). On the other hand, the majority of worms recovered from immunocompetent, reinfected rats were juveniles, comparatively few achieved the mature stage, and they were smaller than those from the primary infected rats. Worms recovered from hamsters showed no morphologic differences with respect to primary infection and reinfection (data not shown).
DISCUSSION
By using the worm recovery rate as a measure of resistance to reinfection, the present study demonstrates for the first time that rats do develop resistance to C. sinensis reinfection. Primary infected rats had a worm recovery rate of more than 50%; however, almost no worms were recovered when they were rechallenged with the same number of metacercariae after the primary infection had been cleared by praziquantel treatment. Worm development was also significantly impaired at reinfection. These findings suggest that the development of acquired protection in rats is the result of pre-exposure to C. sinensis.
However, hamsters failed to develop reinfection resistance, and neither did rabbits (data not shown). Moreover, results on resistance to reinfection in hamsters infected with O. viverrini, another liver fluke closely related with C. sinensis, are controversial (Flavell, 1982; Sirisinha et al., 1983). Flavell (1982) reported that hamsters acquire resistance to O. viverrini, and that this results in a significant worm burden reduction (Flavell, 1982), suggesting that prior infection results in a significant reduction in the faecal egg during a subsequent infection. On the other hand, Sirisinha et al. (1983) demonstrated that a prior infection failed to induce significant protection against reinfection by O. viverrini in hamsters (Sirisinha et al., 1983). A lack of protection was also noted in animals reinfected or superinfected several times with small doses of metacercariae. Thus it is possible that hamsters possess different defense mechanism against C. sinensis or O. viverrini.
In the present study, the mechanism underlying the high resistance to C. sinensis reinfection shown by rats is not clear. However, some observations made during this study suggest the involvement of immune responses in this resistance to reinfection. The resistance rate was found to be weakly related to the intensity of the primary infection (Fig. 2), but this increased when the duration of the primary infection was prolonged sufficiently to allow the worms to mature to the adult stage during the primary infection (Fig. 3). Moreover, resistance to reinfection was substantially suppressed by methylprednisolone (Fig. 4, Table 5), providing further evidence that the resistance may have been due to acquired immunity induced by the primary infection.
Acquired immunity is been known to be closely related with resistance to reinfection by S. mansoni and F. hepatica in rats (Doy et al., 1978; Cetre et al., 1999). After a primary infection, rats develop a strong immunity-mediated resistance to S. mansoni reinfection. Studies have implicated different immune mechanisms, including anaphylactic antibodies (IgE) and non-lymphoid cells, such as eosinophils, macrophages, and platelets, in the rejection of worms between 3 and 4 weeks after a primary infection, and in the development of immunity to superinfection by schistosomes (Capron and Capron, 1986; Khalife et al., 2000). In rat schistosomiasis, a Th2 response was found to benefit the host, and to result in protective immune responses and resistance to reinfection (Cetre et al., 1999). However, this finding is in contrast to observations in a murine model, in which Th2 responses were associated with pathology (Khalife et al., 2000).
In case of F. hepatica, resistance to reinfection in rats is known to occur within 48 hr of challenge, and appears to function at the intestine or body cavity (Doy et al., 1978; Burden et al., 1983). Moreover, acquired resistance has been shown to be associated with anaphylaxis and eosinophilic infiltration of the intestine; eosinophils adhere to newly excysted juveniles of F. hepatica and damage the tegument, when injected intraperitoneally into sensitized rats. However, the mechanism of resistance to C. sinensis may differ from those to F. hepatica and S. mansoni, because of different metacercariae migration patterns in the final hosts in the case of F. hepatica, and because of a different pathogenetic mechanism in the case of S. mansoni.
Recently, strain differences have been reported in mice in terms of their susceptibility to C. sinensis infection (Yoon et al., 2001). FVB/NJ mice were found to be susceptible and BALB/c mice relatively resistant to clonorchiasis (Yoon et al., 2001); moreover, Th2 cytokine response, especially IL-4 production, was associated with the susceptibility to C. sinensis infection (Choi et al., 2003). This suggests that mice may show strain dependent resistance patterns, and that Th2 cytokine response may be associated with resistance to C. sinensis reinfection. In this respect, mice and rats could be useful animal models for investigations of resistance mechanisms to C. sinensis infection.
In the present study, resistance to reinfection was reflected by the delayed development of C. sinensis in reinfected rats, and by a lower worm recovery rate. This contradicts to some extent the findings of Talvik et al. (1998), that challenge by Oesophagostomum quadrispinulatum in pigs produced a lower worm burden, but no apparent stunting and no reduction in fecundity (Talvik et al., 1998). However, suppressed larval development has been observed following repeated infections with Oesophagostomum spp. in pigs (Kendall et al., 1977).
The present study shows that rats developed high levels of resistance to reinfection after clearance of the primary infection, and that this resistance can be substantially impaired by immunosuppression, which suggests the potential role of host immunity in the development of resistance to reinfection. However, the precise nature of the mechanism of this resistance to reinfection requires further investigation. The elucidation of this mechanism would enhance our understanding of the parasite-host relationship, and facilitate the development of effective vaccines for clonorchiasis.
Notes
This study was supported by a Korea Research Foundation Grant (KRF-2003-042-E00034).
