Viability of preserved Cryptosporidium baileyi oocysts
Article information
Abstract
The present study was undertaken to determine the viability and infectivity of oocysts of Cryptosporidium baileyi that had been stored from 1 to 40 months at 4℃ preserved in 2.5% potassium dichromate solution. Oocysts of C. baileyi were purified from the feces of experimentally infected chickens using discontinuous sucrose gradients. Subsequently, the purified oocysts were suspended in 2.5% potassium dichromate solution at a concentration of 1 × 107 organism/ml, and their viabilities were assessed by nucleic acid staining, histologic examination, and infectivity to 2-day-old chickens. All chickens inoculated with oocysts that had been stored for 1-18 months developed patent infections, while chickens infected with older oocysts remained uninfected. Between 5.8% and 82.2% of the oocysts, stored at 4℃ in 2.5% potassium dichromate solution, were found to be viable, as determined by nucleic acid staining. Parasite colonization in the bursa of Fabricius was detected in the microvillus border of bursal epithelium. The finding that C. baileyi oocysts remain infective to chickens for at least 18 months offers important time-saving advantages to investigators who frequently require large numbers of oocysts.
INTRODUCTION
Cryptosporidium baileyi is a coccidian parasite that infects the microvillous regions of the digestive tract, the respiratory tract, and the conjunctiva, causing the loss of epithelial cells and the infiltration of adjacent lamina propria by inflammatory cells. Rhee et al. (1997) reported that C. baileyi infection produces diffuse chronic superficial purulent bursitis with mild to moderate lymphocyte depletion in the lymphoid follicles of bursa of Fabricius (BF) in young chickens. C. baileyi infection has an immunosuppressive effect on Newcastle disease, infectious bronchitis, and on Brucella abortus in young chickens. It is possible that C. baileyi infection suppresses the development of humoral immunity in chicks. Today, Cryptosporidium spp. are ubiquitously distributed in world and the disease has been described in over 79 host species (Mosier and Oberst, 2000). Cryptosporidiosis is recognized as an important food and waterborne disease, which may have significant consequences for public health (Lorenzo-Lorenzo et al., 1993). In birds, cryptosporidiosis can cause respiratory or enteric infections, and the parasite has been reported in over 30 bird species (Sreter and Varga, 2000). Despite the high prevalence and clinical significance of cryptosporidiosis in broiler chickens, no effective chemotherapy, vaccine, or other control method is available (Sreter et al., 1997). Thus, knowledge of factors affecting the survival and infectiveness of Cryptosporidium baileyi oocysts are important with respect to epidemiology and infection control, as well as for the more mundane purpose of storing organisms for research purposes.
Oocysts of Cryptosporidium spp. used for experimental purpose are commonly stored in 2.5% potassium dichromate solution at 4℃. According to Yang et al. (1996), C. parvum oocysts stored under these conditions remain infectious to mice for at least 18 months. Rhee and Park (1996) reported that C. muris oocysts stored at 5℃ lost their infectiveness as early as 6.5 months and that oocysts stored at -70℃ were successfully maintained for 15 months. However, the preservation of C. baileyi oocysts was unsuccessful. Our investigation was undertaken to determine the viability of C. baileyi after the oocysts had been stored for 1-40 months under the conditions described above, in 2-day-old chickens.
MATERIALS AND METHODS
Animals and parasites
In the present study, two-day-old chickens were used. The C. baileyi oocysts used in this study were originally isolated from the domestic chicken, Gallus gallus, and serially passed to 2-day-old SPF chickens after an interval of 2 months (Rhee et al., 1991). To produce oocysts for preservation, 2-day-old SPF chickens were inoculated orally with 1 × 106 C. baileyi oocysts, and oocysts were purified from feces using discontinuous sucrose gradients (Arrowood and Sterling, 1987). The purified oocysts were divided into 20 aliquots (2 × 107 oocysts/aliquot) and stored in 2.5% potassium dichromate solution at 4℃. Prior to infecting chickens, the oocysts were washed with distilled water (DW) to remove the potassium dichromate. To examine the viability of C. baileyi oocysts, 45 chickens were randomly divided into three groups of 15, and housed in different isolation cages (Table 1). The first group of chickens was infected with preserved C. baileyi oocysts, the second group with freshly harvested oocysts, and the third group served as a control and was untreated. Each chicken was inoculated intragastrically with 1 × 106 purified oocysts in a volume of 200 µl of DW. These experiments were performed during the experimental period at intervals of 2 months.
The animals were housed in wire-floored cages, and the cages were placed on trays containing a 5mm depth of 1.8% potassium dichromate solution to prevent feces from drying out. For histologic examination, 5 chickens were sacrificed from each group at 10-days postinoculation (PI).
Viability assay
The viability of preserved oocysts was determined by nucleic acid staining, histology and by their ability to develop patent infections in chickens.
1. Nucleic acid staining
Using the procedure described by Kim and Healey (2001), preserved 1 × 105 oocysts were incubated with 250 u moles the nucleic acid dye SYTO-9 (Molecular Probes, Eugene, Oregon) in microcentrifuge tubes at 37℃ for 60 min in the dark. Oocysts were examined then under fluorescent microscopy, and a total of 200 intact oocysts and/or empty shells were counted: Positive staining represents dead cells. The percentages of viable oocysts were calculated according to the equation: {(number unstained oocysts) ÷ (number oocysts - number empty shells)} × 100. Five determinations were performed per sample.
2. Oocyst shedding in infected chickens
Rhee et al. (1991) reported that a large number of oocysts were found in fecal samples obtained from inoculated chickens on days 8-14 PI. To monitor the oocyst shedding discharge rate, fecal collection from the 10 chickens of all groups was started on day 7 and continued daily until the 14th days PI. The volume of collected fecal samples has not considered because the chickens were removed from their cages and held in a laminar hood to allow collection of all fecal samples. Feces were sieved through a succession of steel mesh screens, the smallest of which had a pore size of 250 µm. Subsequently, oocysts were harvested using discontinuous sucrose gradients, and washed in 0.025 M phosphate buffered saline. The purified oocysts were then resuspended in 2.5% potassium dichromate solution and stored at 4℃ and later counted using a hemocytometer under a microscope.
3. Histologic examination
Chickens were necropsied at day 10 PI. BFs obtained from each bird were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Tissue samples were examined by randomly selecting 5 follicles and counting the number of parasites on each epithelium. The average number of parasites per follicle was then calculated. The index of infection was determine on a scale of 0 to 3 as follows: 0 = no parasite observed; 1 = small numbers of parasites focally distributed in the tissues (10% of the tissue colonized); 2 = moderate numbers of parasites widely distributed throughout the tissues (10% to 50% of the tissue colonized); 3 = large numbers of parasites widely distributed throughout the tissues (> 50% of the tissue colonized).
RESULTS
Fig. 1 shows the viability of preserved oocysts, as determined by nucleic acid SYTO-9 staining. The percentage of live oocysts in the fresh sample was 88.4% ± 2.58 before preservation. After 18 months of preservation at 4℃ in 2.5% potassium dichromate solution, oocyst viability did not decrease, whereas all the oocysts treated at 37℃ for 60 min were dead (data not shown). However, the viability of oocysts preserved for 20 months was significantly reduced (5.8% ± 1.87) versus fresh oocysts (88.4% ± 2.58), 22-40 months old oocysts was 0%.
Viability of preserved Cryptosporidium baileyi oocysts by nucleic acid dye SYTO-9 stain. C. baileyi oocysts had been stored at 4℃ in 2.5% potassium dichromate. Data are presented as the mean + SD.
Infection intensities were determined by quantifying oocyst shedding (Fig. 2). The oocyst shedding intensities of group 1 were not significantly different, from those of group 2. The chickens of group 1, which were infected with 2-18 month preserved oocysts, continued to discharge large numbers of oocysts in their feces during the experimental period, as did chickens of the group 2, but chickens infected with 20-40 months old oocysts and control chickens (uninoculated) remained uninfected.
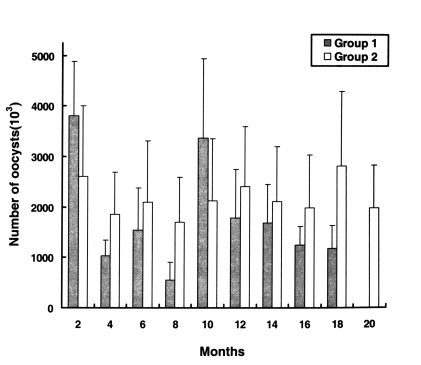
Patterns of Cryptosporidium baileyi oocysts shedding intensity in the chickens of groups 1 (experimental) and 2 (positive control). The first group of chickens was infected with preserved oocysts, the second group with freshly harvested oocysts. Data are presented as the mean + SD.
As shown in Fig. 3, on day 10 PI, the developmental presence of C. baileyi was detected in the microvillous border of the bursal epithelium of chickens in groups 1 and 2. The scores of parasite colonization in the bursa of Fabricius were not different throughout the experimental period. Infection was mediated via heterophils infiltrating the epithelium and adjacent lamina propria. No C. baileyi was observed in the uninoculated chickens.

Parasite colonization of the bursa of Fabricius in the chickens (Groups 1 and 2). The index of infection was determined as follows: 0, no parsite observed; 1, small numbers of parasites (10% of the tissue colonized); 2, moderate numbers of parasites (10 to 50% of the tissue colonized); 3, large numbers of parasites (> 50% of the tissue colonized). Date are presented as the mean + SD.
DISCUSSION
In general, the storage of protozoa at low temperature is a highly effective means of long-term preservation. Although the effects of different preservatives on the viability of C. parvum oocysts have been reported (Robertson et al., 1992), the most commonly used and possibly the best preservative is 2.5% potassium dichromate solution (Fayer et al., 1990), and its effect on the viability of C. parvum oocysts is nor well established. Earlier reports indicated that, while C. parvum oocysts began to loss infectivity at 4 to 6 months when stored at 4℃ in this preservative (Sherwood et al., 1982), others remained viable for 6-9 months (Fayer et al., 1990).
On the other hand, oocysts of C. parvum were infective in immunosuppressed adult C57BL/6N mice after storage for 18 months, at 4℃ in 2.5% potassium dichromate solution (Yang et al., 1996). Rhee and Park (1996) reported that C. muris oocysts stored at 5℃ in 2.5% potassium dichromate solution lost their ability to infect at 6.5 months. However, to the best of our knowledge, no studies on the infectiveness of preserved C. baileyi oocysts have been conducted.
This study shows that infectivity of preserved C. baileyi oocyst was affected by temperature and storage time, and oocysts stored for 18 months under this condition were found to produce patent infections in young chickens.
Yang et al. (2000) reported that a single viable oocyst can induce patent C. parvum infections in immunosuppressed C57BL/6N adult mice, but it is unclear why oocysts stored at 4℃ for 20 months did not infect chickens. Although the number live oocysts after 20 months of storage was significantly reduced, the number of viable oocysts was still high enough to infect chickens. Belosevic et al. (1997) reported that nucleic acid viability assay produces results similar to animal infection experiments. Indeed, compared to other in vitro viability assays, nucleic acid dye staining is a rapid and convenient method for determining protozoan viability. Several methods have been used to examine the viabilities of parasites (Belosevic et al., 1997), however, animal infectivity assays provides the best direct evidence about the ability of a parasite to infect a host.
Observations made in the present study concerning the infectiveness of C. baileyi after storage are in agreement with those made by Yang et al. (1996) about of C. parvum. C. baileyi oocysts in 2.5% potassium dichromate at 4℃ were found to remain infectious to 2-day old broiler chickens after storage for 18 months.
