Specific and common antigens of Clonorchis sinensis and Opisthorchis viverrini (Opisthorchidae, Trematoda)
Article information
Abstract
The antigenic characterizations and serological reactions of human liver flukes, Clonorchis sinensis and Opisthorchis viverrini, were analyzed by immunoblot. The antigenic profiles of the crude extract of Clonorchis contained major proteins of 8, 26-28, 34-37, 43, and 70 kDa, and those of Opisthorchis 34-37, 43, 70, and 100 kDa. Of these, the 8, 26-28 and 34-37 kDa bands of Clonorchis and the 100 kDa of Opisthorchis were major components of each excretory-secretory antigen. The 8 and 26-28 kDa bands were specific to Clonorchis but the 100 kDa of Opisthorchis cross-reacted with the sera of clonorchiasis, and the 34-37, 70 and 100 kDa bands cross-reacted with sera of other helminthiases. The frequency and intensity of the immunoblot reactions were positively correlated with the intensity of the liver fluke infection.
INTRODUCTION
The closely related human liver flukes Clonorchis sinensis and Opisthorchis viverrini are endemic in East and Southeast Asia, respectively (Rim, 1986; Sithithaworn et al., 1994). Infection with these flukes induces severe histopathological changes of the intrahepatic bile duct, causing many complications in chronic and heavily infected cases, such as cholecystitis and cholangiocarcinoma (Rim, 1986; Haswell-Elkins et al., 1992; Sithithaworn et al., 1994). Therefore, they are important helminthic parasites affecting public health in endemic areas.
Although diagnosis of helminthic infections mainly depends on the morphological identification of the eggs in feces, however the differentiation of the eggs between Clonorchis and Opisthorchis among heterophyid trematodes is difficult (Lee et al., 1984). Furthermore, stool examination is labor intensive and may be false-negative in cases with light infections or in the presence of biliary obstruction. Currently people are becoming reluctant to stool examinations, and serodiagnosis is an alternative diagnostic tool, which is useful especially in mass screening of helminthic infections in endemic areas.
Several methods have been developed and evaluated for the serodiagnosis of clonorchiasis (Chen et al., 1994). Among many antigens of C. sinensis, antigens of 10, 28~25, 34 and 43 kDa were reported as target proteins for serodiagnosis of clonorchiasis (Yong et al., 1991; Hong et al., 1997). It was found that the 89 kDa band was a common antigen of the two flukes and the 16 kDa band as a specific antigen of Opisthorchis (Sirisinha et al., 1990). However, their sensitivity and specificity have not been satisfactory yet (Hong, 1988; Hong et al., 1999). In order to find the candidate antigens for serodiagnosis of liver fluke infections, the present study was performed to identify the common and the specific antigens of Clonorchis and Opisthorchis by immunoblot. The reaction frequencies of the major antigens of Clonorchis and Opisthorchis were observed according to the infection density, i.e. EPG (eggs per gram of feces) counts.
MATERIALS AND METHODS
Preparation of crude and excretory-secretory antigens
Metacercariae of Clonorchis sinensis were collected from digested material of the fish, Pseudorasbora parva, caught in the endemic river, Nakdong-gang near Busan, Korea. The metacercariae were introduced into the stomach of New Zealand white rabbits through a gavage tube. The rabbits were bred for 10 weeks at the animal laboratory of the Seoul National University College of Medicine, and fed commercial diet and provided tap water.
Metacercariae of Opisthorchis viverrini were collected from the fish, Puntius orphoides, which were obtained at an endemic reservoir at Khon Kaen in Thailand. Golden hamsters were orally infected with the metacercariae and maintained at the animal laboratory of the Faculty of Medicine, Khon Kaen University for 8 weeks.
Adult worms of Clonorchis or Opisthorchis were recovered from the livers of the rabbits or hamsters after sacrifice by deep anesthesia. Fresh live worms were incubated overnight aseptically at 37℃ in sterile physiologic saline with penicillin/streptomycin (100 units/ml and 100 µg/ml) (Gibco BRL, Grand Island, NY, USA), and they were alive after overnight incubation. The supernatant was used as excretory-secretory (ES) antigen after centrifugation at 15,000 g for 1 hr.
The crude antigens of the liver flukes were prepared by homogenization of adult worms in the presence of E-64 (20 µM, Sigma Co., St. Louis, MO, USA), a cysteine protease specific inhibitor, followed by centrifugation at 15,000 g for 1 hr (Hong et al., 1997). The supernatant was stored at -70℃ until used as crude antigens.
Sera from patients of clonorchiasis or opisthorchiasis
Egg positive cases of Clonorchis were subjected for blood sampling after screening examination using the Kato-Katz method (Garcia and Bruckner, 1997) in endemic areas of Korea and China. The serum specimens of egg positive cases of Opisthorchis were drawn from people in endemic communities in Khon Kaen, Thailand after assessment by quantitative formalin-ethyl acetate concentration technique (Elkins et al., 1991). Total 131 sera of clonorchiasis and 197 opisthorchiasis cases were collected and kept frozen at -70℃ until used. Negative control sera, 10 for clonorchiasis and 31 for opisthorchiasis, were collected from apparently healthy adults residing in nonendemic areas in both Korea and Thailand.
For the cross-reactivity, sera of confirmed human cases of schistosomiasis japonicum, paragonimiasis, metagonimiasis, fascioliasis, gymnophalloidosis, cysticercosis, and sparganosis were screened with Clonorchis and Opisthorchis antigens.
SDS-PAGE and immunoblot
Crude and ES antigens of Clonorchis or Opisthorchis were separated on 7.5-15% polyacrylamide gradient gels under reducing condition. The proteins resolved by SDS-PAGE were transferred onto a PVDF membrane, reacted with sera of clonorchiasis, opisthorchiasis or negative controls in 1:100 dilution, and followed by soaked with peroxidase-conjugated goat anti-human IgG (Cappel, Cochranville, PA, USA) diluted in 1:1,000 (Hong et al., 1997). The final reactions were developed with 4-chloro-1-naphtol and H2O2, and the antigenicity of each protein was observed. The positive rates were determined when one or more specific antigenic bands of Clonorchis or Opisthorchis reacted with sera for each infection.
Determination of EPG
The number of eggs in patients' stools was counted for evaluation of infection density in each infected person by Stoll's method (Garcia and Bruckner, 1997) and expressed as eggs per gram of feces (EPG). Briefly, 4 g of feces were added to bring contents to 60 ml in a Stoll flask containing 56 ml of NaOH, and they were allowed to stand for 24 hr after vigorous shaking with glass beads. The egg number was counted after pipetting out exactly 0.15 ml onto a slide, multipled by 100, and corrected according to the consistency of fecal specimens. EPG counts were repeated three times.
Statistical analysis
Mantel-Haenszel χ2 test was used to evaluate the statistical significances of positive rates, sensitivities and specificities of each antigen between Clonorchis and Opisthorchis.
RESULTS
SDS-PAGE and immunoblot to C. sinensis antigen
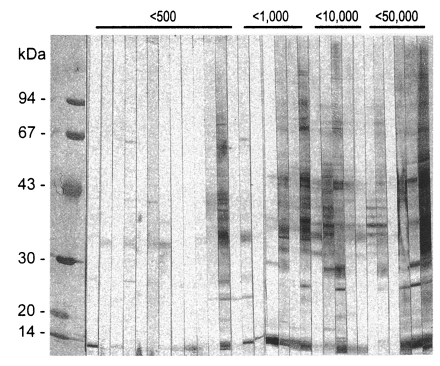
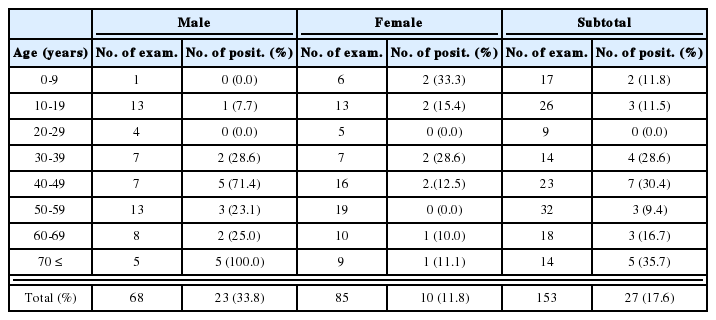
About 20 protein bands from 8 to 100 kDa were separated from the crude antigen of Clonorchis, and less than 10 bands from the ES antigen by SDS-PAGE (Fig. 1). Among the protein bands in the crude antigen, 8, 26-28, 34-37, 43 and 70 kDa proteins were major antigenic proteins, whereas 8, 26-28, 34-37 kDa proteins of ES antigen were antigenic by immunoblot (Fig. 2). The positive rate of immunoblot to crude antigen was 67.2% with sera from clonorchiasis patients, while that of ES antigen was 71.1%. The sensitivity between the crude and ES antigen of Clonorchis was not significantly different (p=0.844). The 34-37 kDa bands showed the highest positive rates followed by 8 kDa band, although the other bands also showed high positive rates (Table 1). The number of bands reacted with sera of clonorchiasis significantly increased as the higher EPG were detected (p = 0.001), and all the sera showing over 2,000 of EPG reacted with one or more antigenic bands (Fig. 3, Table 1).
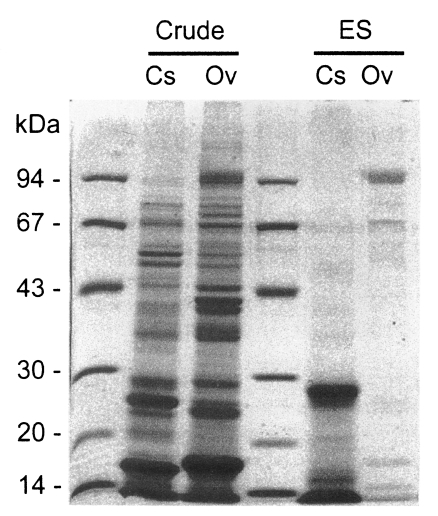
Soluble protein bands separated by SDS-PAGE of crude extracts and excretory-secretory (ES) proteins of Clonorchis sinensis (Cs) and Opisthorchis viverrini (Ov).
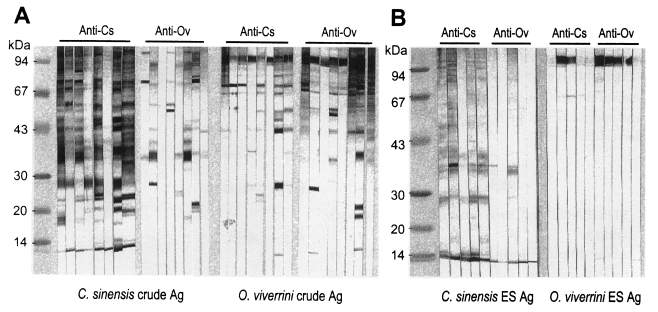
Immunoblot patterns of the crude and ES antigens of Clonorchis sinensis (Cs) and the crude antigen of Opisthorchis viverrini (Ov) with the sera of clonorchiasis or opisthorchiasis subjects.

Reaction frequencies of the major antigenic protein bands of Clonorchis sinensis crude antigen with sera of clonorchiasis and opisthorchiasis classified by intensity of infection
SDS-PAGE and immunoblot to O. viverrini antigen
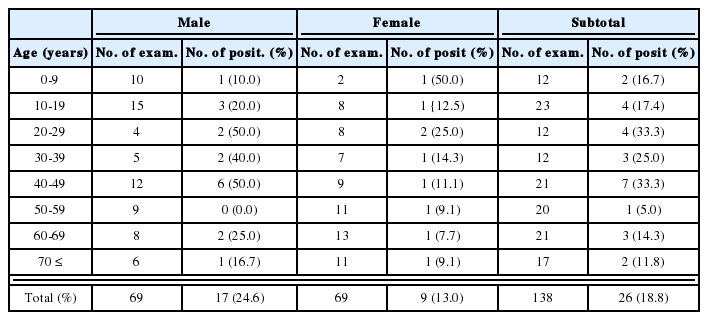
Crude antigen of Opisthorchis was separated into more than 20 protein bands by SDS-PAGE, ranging from 8 to over 100 kDa (Fig. 1). The 100 kDa band was the major protein in the ES antigen, however, other bands of 15, 17, 26, 53 and 70 kDa were also visualized (Fig. 1). On immunoblot, 43, 70 and 100 kDa bands of the crude antigen reacted with the sera from opisthorchiasis patients while only the 100 kDa band in the ES antigen was antigenic (Fig. 2). Of these bands, 70 and 100 kDa bands of crude antigen were major antigens with high frequencies, and the overall positive rate was 93.9%. The sensitivity between the crude and ES antigen of Opisthorchis was not significantly different (p = 0.707). The frequency and the intensity of the immunoblot reactions (data not shown) increased and strengthened by the increase of EPG counts of the opisthorchiasis cases (p = 0.001) (Table 2).
Cross-reactions between Clonorchis and Opisthorchis
The 34-37, 43, 70 and 100 kDa bands of Clonorchis crude antigen cross-reacted with the sera from opisthorchiasis patients (Fig. 2A, Table 1). However, the Clonorchis ES antigen reacted with sera of opisthorchiasis at the 8 and 34-37 kDa bands (Fig. 2B). The crude and ES antigens of Opisthorchis cross-reacted with all of the clonorchiasis sera, especially strongly with the 100 kDa protein (Fig. 2, Table 2).
Cross-reactions with the sera of other helminthiases
The crude antigen of Clonorchis showed cross-reactions with the sera of schistosomiasis, paragonimiasis, fascioliasis, metagonimiasis and cysticercosis, whereas that of Opisthorchis cross-reacted with the sera of schistosomiasis, paragonimiasis, fascioliasis, gymnophalloidosis and cysticercosis (Fig. 4, Table 3). The 34-37 and 70 kDa of Clonorchis crude antigen and 100 kDa of Opisthorchis crude antigen were responsible for the cross-reactions (Fig. 4A). The ES antigen of Clonorchis also cross-reacted with the sera of paragonimiasis, fascioliasis and cysticercosis, and the 34-37 kDa was the major cross-reacting protein (Fig. 4B).
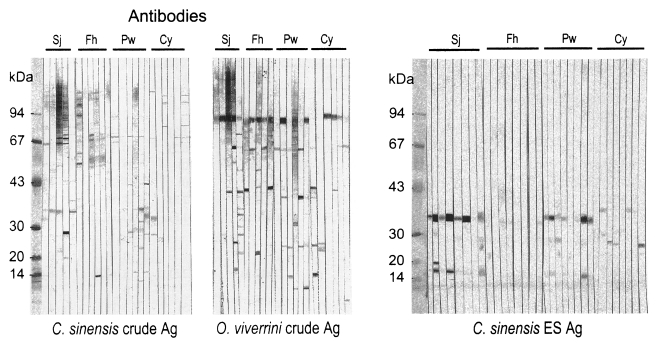
Cross-reactions of the crude or ES antigens of Clonorchis sinensis and the crude antigen of Opisthorchis viverrini with sera of other helminthiasis subjects.
DISCUSSION
Since Clonorchis and Opisthorchis are taxonomically close species, it is expected that they share some common antigenic determinants. The present study shows that they share antigenic protein bands of 34-37, 43, 70 and 100 kDa though the 100 kDa band is not a major antigen of Clonorchis. The 100 kDa band of Opisthorchis reacts more strongly than the other Opisthorchis antigenic proteins and is the only strong one in the ES protein. Furthermore all of the clonorchiasis sera reacted to the 100 kDa band of Opisthorchis. It is too hasty to assume any closely related configuration between the common antigenic molecules of the two liver flukes but they must share common antigenic epitopes.
The major soluble antigenic bands of Clonorchis crude extract are 8, 26-28, 34-37, 43, 70 and 100 kDa, and these crude antigen profiles are in good agreement with those previously described (Hong et al., 1997; Kim, 1998). The 8 and 26-28 kDa bands are major proteins in the ES antigen of Clonorchis. In case of the Opisthorchis crude antigen, the 34-37, 43, 70 and 100 kDa bands were major proteins, and the 100 kDa band was a predominant component in the ES antigen of Opisthorchis. However, it was reported that the 89 kDa protein is a single metabolic product of Opisthorchis (Sirisinha et al., 1990). The present study and that of the Sirisinha et al. (1990) both demonstrated that the antigenic protein band of Opisthorchis is rather simple. However, the 89 kDa band found by Sirisinha et al. (1990) may be the 100 kDa band of the present study because the two were found to be the strongest band of Opisthorchis antigen. The different molecular sizes may be due to differences in the preparation of somatic extracts of Opisthorchis and in the running conditions of SDS-PAGE, however, it is impossible to verify the possible factors of size discrepancy because there were few information to compare with each other. However, in the present study, we did not observe the 16 kDa band reported by Sirisinha et al. (1990).
The ES antigen of Clonorchis or Opisthorchis is relatively rich in major soluble antigenic proteins, and thus it is regarded as a more purified antigen than the crude extract. The sera of clonorchiasis reacted with the 8, 26-28 and 34-37 kDa bands of the ES antigen of Clonorchis and the 100 kDa band of Opisthorchis ES antigen. The sera of opisthorchiasis only reacted with the 100 kDa band of the ES antigen of Opisthorchis. The ES antigen showed slightly higher sensitivity and specificity than the crude antigen in diagnosis of clonorchiasis.
If only the cross-reaction between the two liver flukes is considered, the serodiagnosis would be non-specific. However, since the major antigenic bands are quite different between them, the immunoblot pattern adequately differentiates the two fluke infections. When the serological reaction between the two flukes is neglected and only cross-reaction with other helminthiases is considered, the diagnostic value is much improved. Since the endemic area of Clonorchis and Opisthorchis is geographically separated each other, differential serodiagnosis is not commonly required. In non-endemic areas, however, differentiation is necessary when a serologic reaction is positive to the antigen of either Clonorchis or Opisthorchis.
The serological reaction frequencies of clonorchiasis by immunoblot significantly correlated with the infection intensity, which can be estimated by counting EPGs. The frequencies were as low as 54% among subjects with low EPG (EPG 1-1,000), but 90% of the subjects in the EPG 1,001-2,000 group reacted and almost all of the subjects reacted in those with an EPG exceeding 2,001 (p=0.001) (Table 1). In addition, the serological reaction became stronger as the EPGs increased. This was commonly found in both of Clonorchis and Opisthorchis infections. It has previously been shown that ELISA absorbances are directly related to EPG counts in clonorchiasis (Hong, 1988). The two major Opisthorchis antigenic bands also show 59% frequencies in the EPG 1-1,000 group, but significantly high frequencies in the groups with EPG of over 1,001 (Table 2). As previously recorded in an autopsy study, the EPG count of 1,000 is equivalent to infection of around 62 Opisthorchis worms (Sithithaworn et al., 1991). The present result confirms that the infection of Opisthorchis over 1,001 EPG is an enough burden of worms for serodiagnosis. Another study described a positive correlation with EPGs, worm burden and serum IgG levels by ELISA in opisthorchiasis (Elkins et al., 1991). The study observed highly significant linear correlations between serum IgG levels and EPG counts and also worm burdens, but the serum IgG level varied significantly with age of the subjects.
In this context, the selection of subjects may produce quite different results in serological evaluations. It is strongly recommended, therefore, to define the EPG counts of subjects with clonorchiasis or opisthorchiasis in any serological evaluation. When a study evaluates serological sensitivity of a specific antigen using sera with higher EPG counts than 2,001 or high ELISA absorbances, the result may be an overestimation of the real situation because the majority of clonorchiasis or opisthorchiasis cases encountered in the field are of light infection intensity. Therefore, any serodiagnostic evaluation should consider the reactions of subjects with light infection particularly those with lower EPGs than 1,000. In addition, a serum with a high intensity of infection or strong serological reaction is likely to produce more cross-reactions.
Of the antigenic protein bands of Clonorchis, the 70 kDa band was found to cross-react with sera of several other helminthiases, such as schistosomiasis, fascioliasis, paragonimiasis, metagonimiasis, and cysticercosis. On the contrary, the 8 and 26-28 kDa bands showed no cross-reaction and were Clonorchis specific. One more band of 34-37 kDa from the Clonorchis crude extract also cross-reacted but its frequency was low while that band in the ES antigen reacted more strongly. This stronger reaction may be due to a relatively rich amount of the 34-37 kDa protein in the ES antigen. The cross-reaction of the 35 and 70 kDa bands of Clonorchis with the sera of other helminthiases was already known (Hong et al., 1999), and the 35 and the 34-37 kDa may be the same molecule. Exclusion of the 34-37 and 70 kDa bands from the Clonorchis antigen may improve the specificity of serodiagnosis. Since the 43 and 70 kDa bands are not included in the ES antigen, using the ES antigen improves the specificity in the present study.
Of the Opisthorchis antigenic protein bands, both 70 and 100 kDa bands cross-reacted with sera of other helminthiases. Especially the 100 kDa band, which is the major antigenic protein of Opisthorchis, cross-reacted strongly with the sera of schistosomiasis, paragonimiasis, fascioliasis and cysticercosis as well as that of clonorchiasis. The 100 kDa band of Opisthorchis may be beset with the common antigenic epitope of many human flukes, which is an interesting point of further study. Therefore, it is hard to designate any specific antigen of Opisthorchis for serodiagnosis.
In conclusion, the 8 and 26-28 kDa antigenic bands of Clonorchis was found to be specific for serodiagnosis of Clonorchiasis, whereas no specific antigen of Opisthorchis were observed for serodiagnosis of Opisthorchiasis. Clonorchis and Opisthorchis share common antigens observed at 34-37, 43, 70 and 100 kDa in molecular mass, which cross-reacted with the sera of several other helminthiases. Since the ES antigen of Clonorchis includes rich antigenic bands without the 43 and 70 kDa bands, it may improve the sensitivity and specificity of serodiagnosis to 71.1% and 91.1% while those of the crude antigen are 68.8% and 83.5% respectively. The frequency and the intensity of the serological reaction are positively correlated with the infection intensity of Clonorchis or Opisthorchis and most of the cases over EPGs 2,001 reacted with the antigenic bands. The EPG counts of sampled human sera should be considered in any evaluation of serodiagnostic findings.
ACKNOWLEDGMENTS
We thank Professor Seung-Yull Cho (Department of Molecular Parasitology, Sungkyunkwan University College of Medicine) for donating the human sera of several helminthiases to this study.
Notes
This study was supported by a grant for international cooperative research from the Korea Science and Engineering Foundation (#KOSEF 965-0716-001-2).