Usefulness of 8 kDa protein of Fasciola hepatica in diagnosis of fascioliasis
Article information
Abstract
This study was designed to detect and evaluate an antigenicity of low molecular weight proteins of Fasciola hepatica in fascioliasis. Low molecular weight protein of F. hepatica was purified by ammonium sulfate precipitation and Sephacryl S-100 HR gel filtration. The protein obtained was estimated to be 8 kDa on 7.5-15% gradient sodium dodecyl sulfate gel electrophoresis. Immunoblotting studies showed that the 8 kDa protein reacted with human fascioliasis sera, but not other trematodiasis sera. This result suggests that the 8 kDa protein of F. hepatica is one of diagnostic antigens in human fascioliasis without cross-reaction with other human trematodiasis.
Fascioliasis is an important zoonotic disease caused by the liver fluke, Fasciola hepatica, and is found world-wide in sheep and cattle. Humans can be accidentally infected by ingesting water plants contaminated with metacercariae (Beaver et al., 1984). The diagnosis of human fascioliasis is performed by detection the eggs in stools; however, immunodiagnosis including enzyme-linked immunosorbent assay (ELISA) and western blotting is now widely applied (Hillyer, 1998). It is well documented that several antigens such as fatty acid binding protein and cathepsin L1 cysteine protease are useful diagnostic molecules (Hillyer, 1998; O'Neill et al., 1998, 1999). In order to evaluate diagnostic antigenicity of the low molecular weight proteins of F. hepatica, the authors purified 8 kDa protein and evaluated its antigenic properties in part.
Adult worms of F. hepatica were collected from bile ducts of naturally infected cattle. The parasites were washed three times with sterile saline. The crude extracts of the parasites were prepared using Teflon-pestle homogenizer in 50 mM Tris-HCl buffer (pH 7.5) containing 0.2 mM iodoacetamide to prevent proteolytic degradation (Chung et al., 2002). The homogenates were centrifuged at 15,000 rpm for 40 min and the resulting supernatants were used as the crude extracts. The 8 kDa protein was purified as described previously (Chung et al., 2002). Briefly, the crude extracts were saturated with ammonium sulfate and the resulting supernatants were separated through Sephacryl S-100 HR column equipped with FPLC system (Amersham pharmacia Biothech, Uppsala, Sweden). Eluted fractions were monitored by 7.5-15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To evaluate diagnostic antigenicity of the purified 8 kDa protein, immunoblotting was performed. Purified protein bands on gels were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Stripped membranes were incubated overnight with 1:100 diluted human trematodiasis sera and then incubated with 1:1,000 diluted peroxidase conjugated anti-human IgG (Cappel, PA, USA) for 2 hr. The blots were visualized with 4-chloro-1-naphtol (Sigma, St. Louis, MO, USA) and stopped with doubly distilled water. Human fascioliasis sera confirmed by ELISA and western blotting were kindly donated by Professor Seung-Yull Cho.
The purification process of the 8 kDa protein was shown in Fig. 1. The supernatant fractions after ammonium sulfate precipitation contained 28, 17, 8 kDa and 7 kDa protein bands (lane 2). When the supernatant fraction was further purified through Sephacryl S-100 HR gel filtration (lane 3), the fractions with molecular weight of about 110 kDa as estimated by Sephacryl S-100 HR gel filtration chromatography (data not shown) migrated at 8 kDa on 7.5-15% gradient SDS-PAGE (lane 3). Similar process of purification was reported in 8/7 kDa protein of Clonorchis sinensis, of which molecular weight was estimated as 110 kDa in Superose 6 HR chromatography (Chung et al., 2002).

SDS-PAGE analysis of the purified 8 kDa protein from Fasciola hepatica crude extracts. The proteins were analyzed on 7.5-15% gradient SDS-PAGE. Lane 1, crude extracts; lane 2, supernatants of ammonium sulfate saturation; lane 3, purified 8 kDa protein.
Immunoblot analysis showed that the 8 kDa protein in the crude extracts reacted positively with human fascioliasis sera (Fig. 2A). In the crude extracts of F. hepatica, proteins of various molecular weight reacted with the sera in addition to 8 kDa protein. Of five fascioliasis sera, four reacted positively against the purified 8 kDa as much as to that in the crude extracts (Fig. 2B). The healthy sera did not reacted against F. hepatica proteins. In order to evaluate cross-reactivity of the 8 kDa, immunoblottings were done using sera from paragonimiasis, clonorchiasis and schistosomiasis japonica patients. As shown in Fig. 3, the 8 kDa protein did not react with other trematodiasis human sera.

Immunoblotting of crude extracts and the 8 kDa protein with human fascioliasis sera. (A) Crude extracts of Fasciola hepatica. (B) purified 8 kDa. Strip a-e, human fascioliasis sera; f and g, healthy sera. Electrophoresis was performed on 7.5-15% gradient gel. The arrow indicates the reactive 8 kDa.
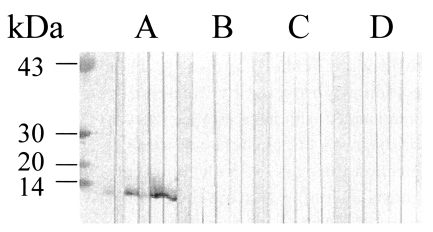
Immunoblotting of the purified 8 kDa protein with trematodiasis patients sera. (A) Human fascioliasis sera. (B) paragonimiasis sea. (C) clonorchiasis sera. (D) schistosomiasis japonica sera.
In the present study, we purified a 8 kDa protein from F. hepatica and showed that this protein is reactive against human fascioliasis sera. Although the biochemical nature and function of this protein are yet to be known, this 8 kDa protein of F. hepatica showed similar biochemical properties with the 8 kDa protein of C. sinensis with respects to its solubility at high ammonium sulfate solution, its molecular size and its mobilities by column chromatography and SDS-PAGE. It is felt likely that the protein with similar biochemical characters may be a commonly present antigenic protein in different trematodes. Species-specific antigenicity of these 8 kDa proteins are warranted further studies especially as diagnostic antigen.
ACKNOWLEDGMENTS
Authors deeply thank Professor Seung-Yull Cho for providing human fascioliasis sera and also thank Professor Sung-Tae Hong for providing human schistosomiasis sera.