Effects of anti-allergic drugs on intestinal mastocytosis and worm expulsion of rats infected with Neodiplostomum seoulense
Article information
Abstract
The effects of anti-allergic drugs on intestinal mastocytosis and the expulsion of Neodiplostomum seoulense were observed in Sprague-Dawley rats, after oral infection with 500 metacercariae. The drugs used were hydroxyzine (a histamine receptor H1 blocker), cimetidine (a H2 blocker), cyclosporin-A (a helper T-cell suppressant), and prednisolone (a T- and B-cell suppressant). Infected, but untreated controls, and uninfected controls, were prepared. Worm recovery rate and intestinal mastocytosis were measured on weeks 1, 2, 3, 5, and 7 post-infection. Compared with the infected controls, worm expulsion was significantly (P < 0.05) delayed in hydroxyzine- and cimetidine-treated rats, despite mastocytosis being equally marked in the duodenum of all three groups. In the cyclosporin-A- and prednisolone-treated groups, mastocytosis was suppressed, but worm expulsion was only slightly delayed, without statistical significance. Our results suggest that binding of histamine to its receptors on intestinal smooth muscles is more important in terms of the expulsion of N. seoulense from rats than the levels of histamine alone, or mastocytosis.
INTRODUCTION
Neodiplostomum seoulense (Digenea: Neodiplostomidae) is an intestinal fluke of humans and rodents in the Republic of Korea (Seo, 1990). The grass snake, Rhabdophis tigrina, a paratenic host, is an important source of human infection (Hong et al., 1982; Seo, 1990). The adult flukes parasitize the duodenum of rodents and, if worm burdens are high, they extend to the jejunum and ileum (Hong, 1982). In experimentally infected rodents, villous atrophy (including a decreased villus-crypt ratio, blunting and fusion of the villi), and crypt hyperplasia (with stromal inflammations) were found to be the major histopathological changes in the small intestine (Lee et al., 1985). Infection with this trematode can be lethal to mice (Huh et al., 1988; Kook et al., 1998; Chai et al., 2000), and infected people may suffer from symptoms, which include diarrhea, abdominal pain, and fever (Seo, 1990).
In rats infected with N. seoulense, the worm recovery rate (WRR) decreases from four weeks post-infection (PI) (Hong et al., 1983). Mucosal mast cells (MMCs), which increased markedly in the duodenum at around week 3 PI, were proposed to be an important effector of this spontaneous worm expulsion (Kho et al., 1990). In mice, however, MMCs were found to play only a minor role in the expulsion of N. seoulense (Chai et al., 1998). Therefore, the mechanisms of N. seoulense expulsion may be different in rats and mice, and the role of MMCs in rats requires verification.
In this study, we investigated the effects of four types of anti-allergic drugs on mastocytosis and on the worm expulsion of N. seoulense from the rat small intestines. These drugs examined were, prednisolone (a T- and B-cell suppressant), cyclosporin-A (a helper T-cell suppressant), hydroxyzine (a histamine receptor H1 blocker), and cimetidine (a H2 blocker).
MATERIALS AND METHODS
Collection of the metacercariae and the experimental infection of rats
The metacercariae of N. seoulense were collected from 50 grass snakes (Rhabdophis tigrina), which were purchased from a local snake collector in Jinju, Gyeongsangnam-do, using an artificial digestion technique. A total of 185 Sprague-Dawley rats 3-week-old were purchased from the Laboratory Animal Center, at Seoul National University. The animals were treated twice with mebendazole (10 mg/kg) and praziquantel (25 mg/kg), and confirmed to be free of helminth eggs by fecal examinations before being used in the experiment.
The rats were divided into 4 drug-treated and 2 control groups; i.e., hydroxyzine-, cimetidine-, cyclosporin-A- and prednisolone-treated, and uninfected and infected but untreated controls. Each group was composed of 30 rats, but 5 rats were added to the prednisolone-treated group because prednisolone treatment was expected to affect rat survival during the experiment. Each rat in the infected groups was fed 500 metacercariae of N. seoulense orally using a gavage needle. The uninfected controls were given only water through the same needle type.
Drug administration
Hydroxyzine, at a daily dose of 1 mg/kg, and cimetidine, at a daily dose of 20 mg/kg, were diluted in water and administered orally from the day of infection until sacrifice. Cyclosporin-A, which is insoluble in water, was diluted initially in a small amount of absolute ethanol; this solution was then diluted with water just prior to use, and administered orally at a daily dose of 7.5 mg/kg, until sacrifice.Prednisolone (10 mg/kg) was injected intramuscularly every other day into the inner thighs of the rats, until sacrifice.
Histochemistry for MMCs and worm recovery
Rats were sacrificed on weeks 1, 2, 3, 5 and 7 PI by cervical dislocation. Abdomens were incised, and intestinal segments, about 1.5 cm in length, were taken from the middle portion of the duodenum, jejunum, and ileum. The segments were washed with saline 2-3 times, and fixed in Carnoy's fixative for 6 to 12 hr. The fixed tissues were then embedded in paraffin, and sectioned at 4 µm. The tissue sections obtained were stained with 1% alcian blue (Janssen Pharmaceutica, Belgium) (pH = 0.3), and counterstained with safranin O (pH = 1.0) (Merck, USA), as described by Strobel et al. (1981). The number of MMCs in a defined region of 10 villus-crypt units (VCU) was counted for each stained sample, and data are expressed as means and SD of the number of MMCs per VCU.
Adult flukes of N. seoulense were recovered from the segments of the small intestines, using Baermann's apparatus (Chai et al., 1998). Worms were collected from the bottom of a test tube, and counted under a stereomicroscope to determine the WRRs. The WRR was calculated for each rat by dividing the number of recovered worms by the number of metacercariae given.
Statistical test
Statistical significance between the groups was determined by using the paired t-test. Values of P < 0.05 were considered significant.
RESULTS
Worm recovery
In the infected controls, the WRRs did not change significantly over weeks 1-3 PI, ranging from 33.6 ± 6.2% to 38.4 ± 5.5%, but they decreased significantly (P < 0.05) to 23.6 ± 5.2% and 19.9 ± 8.0%, at weeks 5 and 7 PI, respectively (Fig. 1). The four drug treatment groups had similar chronological patterns. However, throughout the observation period, the WRRs were significantly (P < 0.05) higher in hydroxyzine- and in cimetidine-treated rats, than in the infected controls (P < 0.05 except at week 2 PI; Fig. 1). The WRRs of the cyclosporin-A- and prednisolone-treated groups during weeks 1 and 7 PI were slightly higher than in the infected controls; however, this difference was only statistically significant (P < 0.05) at week 5 PI for the cyclosporin-A-treated animals (Fig. 1).
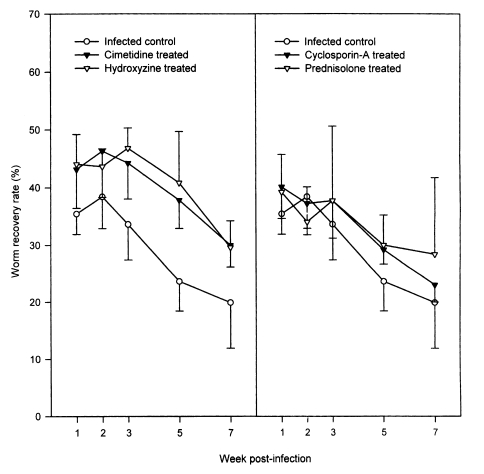
Worm recovery of Neodiplostomum seoulense in rats treated with anti-allergic drugs versus infected controls. Each rat was infected with 500 metacercariae, and sacrificed at weeks 1-7 post-infection (PI). The worm recovery rates (WRRs) were significantly higher in hydroxyzine-, and cimetidine-treated rats compared with the controls (P < 0.05; except at week 2 PI). The WRRs in cyclosporin-A- and prednisolone-treated groups were slightly higher than in the infected controls; however, statistical significance was observed only at week 5 PI between the cyclosporin-A-treated animals and the infected control group (P < 0.05).
Changes in the number of MMCs in the rat intestine
In the duodenum of uninfected control rats, the number of MMCs per VCU ranged from 11.5 ± 3.3 (at week 2 PI) to 15.6 ± 4.0 (at week 5 PI), and showed little variation (Fig. 2). In the jejunum and ileum, MMC numbers were almost identical to those of the duodenum of the uninfected controls. In N. seoulense-infected rats, however, the numbers of duodenal MMCs per VCU were greater, 18.9 ± 5.5 at week 1 PI, and this increased rapidly and remarkably to 41.6 ± 11.4 and 68.9 ± 21.7 at weeks 2 and 3 PI, respectively (Fig. 2). After peaking at week 3 PI, the number of duodenal MMCs decreased gradually to 53.7 ± 10.9 and 42.3 ± 10.4, at weeks 5 and 7 PI, respectively (Fig. 2). On the other hand, in both the jejunum and ileum of rats infected with N. seoulense, MMC numbers increased only slightly, but significantly (P < 0.05), throughout the infection period (Fig. 2).
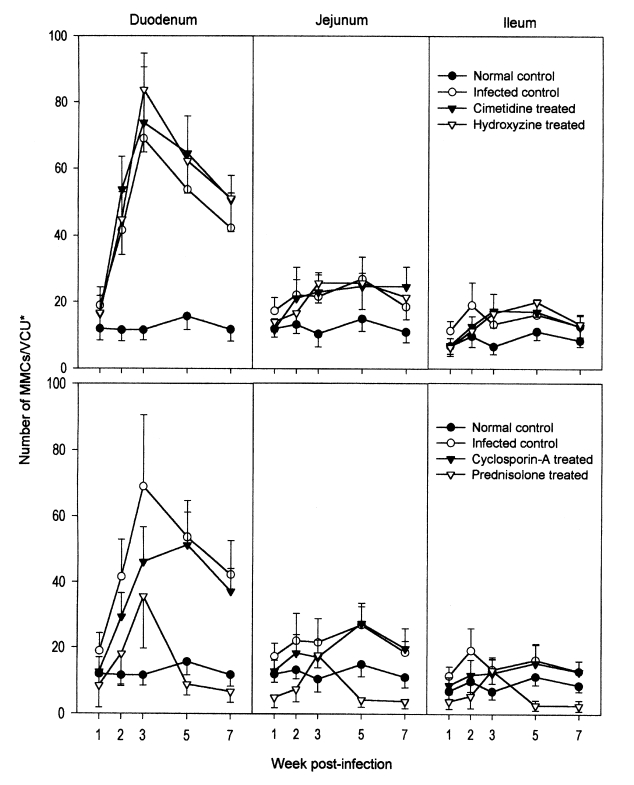
Mucosal mast cell (MMC) responses in the small intestines of rats, after an infection with Neodiplostomum seoulense and treatment with anti-allergic drugs. Data are expressed as the numbers of MMCs per villus-crypt unit (VCU*). In N. seoulense-infected rats, the number of duodenal MMCs peaked at week 3 PI, and then decreased gradually. Jejunal and ileal MMCs increased only slightly through the infection period. In hydroxyzine- and cimetidine-treated rats, mastocytosis levels in the duodenum were similar to that of the infected controls. By comparison, in rats treated with cyclosporin-A or prednisolone, mastocytosis was lower than in the infected controls. In the jejunum and ileum, mastocytosis was unremarkable in all drug-treated animals.
In the duodenums of hydroxyzine- and cimetidine-treated rats, the chronological patterns of mastocytosis were similar to those of the infected controls (Fig. 2). MMC numbers were slightly greater in the drug-treated groups than in the untreated controls; however, this was not statistically significant (P > 0.05). The chronological patterns of MMCs in the jejunum and ileum were similar for hydroxyzine- and cimetidine-treated animals and infected controls (Fig. 2).
Different chronological patterns of MMCs were observed in the cyclosporin-A- and prednisolone-treated groups (Fig. 2). In the duodenum of the cyclosporin-A-treated group, the number of MMCs increased gradually and peaked at week 5 PI, and then decreased from week 7 PI (Fig. 2). However, compared with the infected controls, the number of MMCs during weeks 1-3 PI was significantly (P < 0.05) lower in the cyclosporin-A-treated group (Fig. 2). In the duodenum of prednisolone-treated rats, the number of MMCs increased gradually and peaked at week 3 PI, and then decreased at weeks 5 and 7 PI (Fig. 2). However, the peak level of mastocytosis in the prednisolone-treated group was only a half of that of the infected controls and of the hydroxyzine- and cimetidine-treated groups; MMC numbers through weeks 1-7 PI were significantly (P < 0.05) lower than in the infected controls (Fig. 2). In the jejunum and ileum of cyclosporin-A- and prednisolone-treated rats, mastocytosis was unremarkable through weeks 1-7 PI (Fig. 2).
DISCUSSION
Host-parasite relationships related to the expulsion mechanisms of helminths from the rodent intestine have been studied using a variety of nematode and trematode models (Chai et al., 1993; Fujino et al., 1998; Koyama and Ito, 2000). Reviews of these studies have led to the conclusion that intestinal MMCs and goblet cells (GCs) are the two major effector cell types (Else and Finkelman, 1998; Onah and Nawa, 2000).
The relative importances of the effector cell are, however, different and depend on parasite species. For example, MMCs, but not GCs, play a vital role in the expulsion of Strongyloides ratti, whereas GCs, but not MMCs, play an essential role in the expulsion of N. brasiliensis and Echinostoma trivolvis from rodents (Nawa et al., 1994; Fujino et al., 1998; Onah and Nawa, 2000). It has also been shown that MMCs are not essential for the protection of mice against infection with the nematode, Trichuris muris (Koyama and Ito, 2000), or for protecting rats against infection with the cestode, Hymenolepis diminuta (Starke and Oaks, 2001). Moreover, mucosal mastocytosis was suppressed by unknown reasons in a nematode infection with Nematospiroides dubius in mice (Dehlawi et al., 1987).
In the case of N. seoulense infection, spontaneous worm expulsion, in association with intestinal mastocytosis (notably in the duodenum), has been observed in rats (Kho et al., 1990), and in mice (Chai et al., 1998). The kinetics and extent of mastocytosis, however, were different between the two animal hosts. In rats, the number of MMCs peaked at week 3 PI, and then decreased gradually up to week 7 PI (Kho et al., 1990); but in mice, it peaked at week 1 PI, and quickly decreased to the normal level at week 2 PI (Chai et al., 1998). The peak MMC number in rats was about 80 per VCU (Kho et al., 1990), 4~5-fold higher than the peak value in BALB/c mice (Chai et al., 1998). Therefore, the role of MMCs in the expulsion of N. seoulense may be different for mice and rats. Consequently, in mice, neither MMCs nor GCs were shown to play a major role in worm expulsion by BALB/c and C3H mice (Chai et al., 1998), whereas the role of MMCs remains to be elucidated in rats (Kho et al., 1990). In this study, rats were used as the experimental host, and worm expulsion with intestinal mastocytosis was observed; nevertheless, the importance of MMCs in worm expulsion is difficult to determine.
In relation to the actions of MMCs, the enzymes produced by these cells, which include amines like histamine and serotonin, as well as proteases and peroxidases, are candidate biochemical mediators responsible for intestinal nematode expulsion (Woodbury et al., 1984). In particular, histamine is known to enhance intestinal smooth muscle contraction, which could facilitate worm expulsion, by binding to histamine receptors (Mary et al., 2000). The present study supports for the importance of histamine in worm expulsion, because treatments with hydroxyzine or cimetidine, antagonists of the histamine receptors, significantly delayed worm expulsion from rats. Hydroxyzine is known to block the H1 receptor (Mary et al., 2000), and thus inhibits the action of histamine and worm expulsion, regardless of the level of mastocytosis. Similarly, mepyramine, another H1 antagonist, inhibits the expulsion of Trichostrongylus colubriformis from guinea pigs (Rothwell et al., 1978). Cimetidine has the ability to inhibit histamine-induced smooth muscle contraction (Scott and Maric, 1993), by blocking the histamine receptor H2, and thus can inhibit worm expulsion regardless of the level of mastocytosis.
In this study, however, the effects of cyclosporin-A and prednisolone on the expulsion of N. seoulense are unclear. Cyclosporin-A has a reversible immunosuppressive effect on helper T-cells (McLauchlan et al., 1999), and prednisolone reduces mast cell numbers and mast cell protease levels dose-dependently (King et al., 1985; Goldsmith et al., 1990); both inhibit mastocytosis in the host intestinal mucosa. Unexpectedly, however, the WRRs in cyclosporin-A- and prednisolone-treated rats were only slightly higher than that in infected controls; this difference was not statistically significant. Mastocytosis was markedly depressed in both drug-treated groups. This discrepancy strongly suggests the possible existence of effectors other than MMCs and histamine for the expulsion of N. seoulense from rats. Intestinal GC is a candidate effector, as has been reported in other trematode species, such as E. trivolvis (Weinstein and Fried, 1991) and E. caproni (Fujino et al., 1996). In mice, GCs have been shown to play only minor roles in BALB/c and C3H strains in the expulsion of N. seoulense (Chai et al., 1998), however, their role in rats needs to be clarified.
In this connection, it is of considerable interest to note that histamine can stimulate airway GC secretion in guinea pigs, and that cimetidine, an antagonist of histamine H2 receptor, can abolish this mucus response (Takeyama et al., 1996; Tamaoki et al., 1997). We speculate, therefore, that cimetidine, and possibly hydroxyzine affect the histamine receptors on GCs in the intestinal tracts of rats, and suppress the action of GCs. Information upon the effects of histamine on intestinal GCs, however, is lacking. Studies on the effects of antihistamines on the intestinal GC responses of rats infected with N. seoulense are required.
Notes
This study was supported by a grant from the Seoul National University College of Medicine Research Fund (2000).