Review of Neospora caninum and neosporosis in animals
Article information
Abstract
Neospora caninum is a coccidian parasite of animals. It is a major pathogen for cattle and dogs and it occasionally causes clinical infections in horses, goats, sheep, and deer. Domestic dogs are the only known definitive hosts for N. caninum. It is one of the most efficiently transmitted parasite of cattle and up to 90% of cattle in some herds are infected. Transplacental transmission is considered the major route of transmission of N. caninum in cattle. Neospora caninum is a major cause of abortion in cattle in many countries. To elicit protective immunity against abortion in cows that already harbor a latent infection is a major problem. This paper reviews information on biology, diagnosis, epidemiology and control of neosporosis in animals.
INTRODUCTION
Since its first recognition in 1984 in dogs in Norway (Bjerkås et al., 1984) and the description of a new genus and species Neospora caninum (Dubey et al., 1988a), neosporosis has emerged as a serious disease of cattle and dogs worldwide. Additionally, clinical neosporosis has been reported in sheep, goats, deer, a rhinocerus, and horses and antibodies to N. caninum have been found in the sera of water buffaloes, red and gray foxes, coyotes, and camels, and felids.
BIOLOGY
Life Cycle
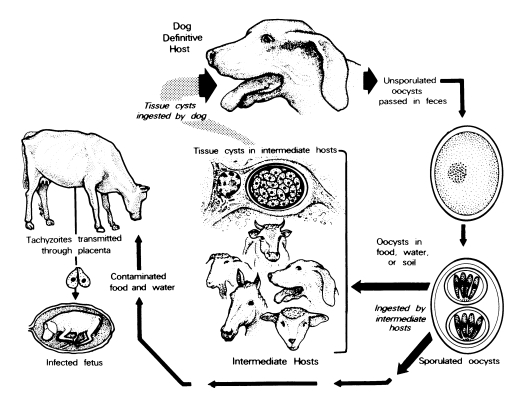
Dogs are both the intermediate and definitive host for N. caninum (McAllister et al., 1998; Lindsay et al., 1999a, 1990b, 2001a; Basso et al., 2001a; Dubey et al., 2002). The life cycle is typified by 3 infectious stages: tachyzoites, tissue cysts, and oocysts (Fig. 1 and Fig. 2). Tachyzoites and tissue cysts are the stages found in the intermediate hosts and they occur intracellularly (Dubey et al., 2002). Tachyzoites are approximately 6 × 2 μm (Fig. 1). Tissue cysts are often round or oval in shape, up to 107 μm long and are found primarily in the central nervous system (CNS). The tissue cyst wall is up to 4 μm thick and the enclosed bradyzoites are 7-8 × 2 μm. Thin-walled (0.3-1.0 μm) tissue cysts have been recently reported in muscles of cattle and dogs naturally-infected with a N. caninum-like parasite (Peters et al., 2001a). These intramuscular tissue cysts have not been yet found in experimentally-infected animals (Dubey et al., 2002).
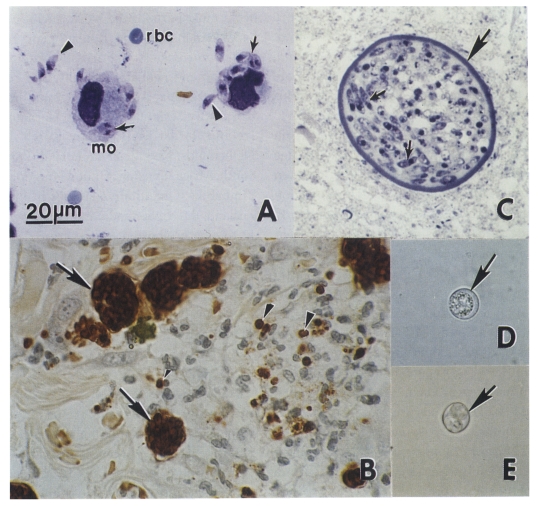
Neospora caninum stages in dogs. Bar = 20 µm and applies to all figures. (A) Tachyzoites in an impression smear of lung. Giemsa stain. Note individual organisms (arrowheads) and those dividing into 2 (arrows). Compare size with red blood cells (rbc) and a macrophage (mo). (B) Tachyzoites in groups (arrows) and individuals (arrowheads) in sections of skin. Immunohistochemical stain with anti-N. caninum antibody. (C) Tissue cyst in section of brain. Note thick tissue cyst wall (arrow) enclosing bradyzoites (arrows). Toluidine blue stain. (D) Unsporulated oocyst with an individual sporont (arrow). Unstained. (E) Sporulated oocyst (arrow). Unstained.
Domestic dogs are the only known definitive host for N. caninum. N. caninum unsporulated oocysts from experimentally-infected dogs were 11.7 × 11.3 (10.6-12.4 × 10.6-12.0) μm in size (Lindsay et al., 1999a). N. caninum oocysts sporulate outside the host. N. caninum oocysts are morphologically similar to Toxoplasma gondii and Hammondia hammondi oocysts in cat feces and broadly resemble oocysts of Hammondia heydorni-like parasite in dog feces (Dubey et al., 2002). At the present, nothing is known regarding the frequency of shedding of oocysts, the survival of the oocysts in the environment, and whether other canids are also definitive hosts for N. caninum.
Transmission
Little is known about the mode of spread and tissue distribution of N. caninum in animals using the natural routes of transmission. The parasite can be transmitted transplacentally in several hosts and vertical route is the major mode of its transmission in cattle. Carnivores can acquire infection by ingestion of infected tissues (McAllister et al., 1998; Lindsay et al., 1999a, 1999b; Dijkstra et al. 2001; Schares et al., 2001; Gondim et al., 2002).
It is epidemiologically important to be able to identify N. caninum oocysts in dog feces. Microscopic examination alone will not be enough to identify N. caninum oocysts in dog feces. Methods have also been developed to genetically distinguish N. caninum oocysts from H. heydorni oocysts (Hill et al., 2001; Šlapeta et al., 2002). Neospora caninum oocysts have been identified feces of only two naturally-infected dogs (Basso et al., 2001a; Šlapeta et al., 2002).
Animal models
There are no suitable animal models at the present to perform bioassay to detect N. caninum oocysts in dog feces. Although interferon-gamma gene knockout (KO) mice are highly susceptible to parenteral inoculation with N. caninum tachyzoites and tissue cysts (Dubey and Lindsay, 1996; Dubey et al., 1998a), they are less susceptible to parenteral or oral inoculation with oocysts. Gerbils (Meriones unguiculatus) were susceptible to N. caninum infection with oocysts (Dubey and Lindsay, 2000; Basso et al., 2001a; Schares et al., 2001). Another species of gerbils, Meriones tristrami and sand rats (Psammoomys ubesus) also susceptible to tachyzoites infection (Pipano et al., 2002).
Comparison between neosporosis and toxoplasmosis
A large body of knowledge has been generated concerning biology of N. caninum because of previous experience with the related parasite, Toxoplasma gondii. Although N. caninum and T. gondii are very closely related parasites structurally, genetically, and immunologically, caution should be used in making generalizations about N. caninum based on the biology of T. gondii because neosporosis and toxoplasmosis are biologically distinct diseases. Toxoplasma gondii is a major disease of sheep and humans and not of cattle, whereas neosporosis is a major disease in cattle, not of sheep and there is no evidence for human infection.
NEOSPOROSIS IN CATTLE
Bovine neosporosis has been reviewed in several papers (Dubey and Lindsay, 1996; Wouda, 1998; Dubey, 1999; Anderson et al., 2000; Buxton et al., 2002; Dijkstra, 2002; Innes et al., 2002; Jenkins et al., 2002; Dubey, 2003). Therefore, most references on bovine neosporosis were omitted from this review.
Clinical signs
Neospora caninum causes abortion both dairy and beef cattle. Cows of any age may abort from 3 month gestation to term. Most neosporosis-induced abortions occur at 5-6 month gestation. Fetuses may die in utero, be resorbed, mummified, autolyzed, stillborn, born alive with clinical signs, or born clinically normal but chronically infected. Neosporosis-induced abortions occur year-round. Cows with N. caninum antibodies (seropositive) are more likely to abort than seronegative cows and this applies to both dairy and beef cattle. However, up to 95% of calves born congenitally-infected from seropositive dams remain clinically normal. The age of dam, lactation number, and history of abortion generally do not affect rate of congenital infection but there are reports indicating that in persistently infected cattle vertical transmission is more efficient in younger than older cows.
Clinical signs have only been reported in cattle younger than 2 month of age. Neospora caninum-infected calves may have neurologic signs, be underweight, unable to rise, or be born without clinical signs of disease. Hind limbs or forelimbs or both may be flexed or hyperextended. Neurologic examination may reveal ataxia, decreased patellar reflexes, and loss of conscious proprioception. Calves may have exophthalmia or asymmetrical appearance in the eyes. Neospora caninum occasionally causes birth defects including hydrocephalus and narrowing of the spinal cord.
Abortions may be epidemic or endemic (Wouda et al., 1999a). In infected areas, as many as 33% of dairy cow fetuses have been reported to abort within a few months. Abortions were considered epidemic if more than 10% of cows at risk aborted within 6-8 weeks. A small proportion (< 5%) of cows have been reported to have repeated abortion due to neosporosis (Anderson et al., 1995).
Prevalence
Neospora caninum infections have been reported from most parts of the world including Australia, New Zealand, Europe, Korea, Japan, Thailand, and the Americas. Neosporosis-associated bovine abortion and neonatal mortality has been reported from Argentina, Australia, Belgium, Brazil, Canada, Costa Rica, Denmark, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Korea, Mexico, the Netherlands, New Zealand, Poland, Portugal, Spain, South Africa, Sweden, United Kingdom, USA, and Zimbabwe. Quantitative studies in the USA, New Zealand, the Netherlands, and Germany indicate that 12 to 42% of aborted fetuses from dairy cattle are infected with N. caninum (Table 1).
Serologic prevalence in cattle varies, depending on the country, region, type of serologic test used, and cut-off level used to determine the exposure. In some dairies up to 87% of cows are seropositive.
Diagnosis
Examination of the serum from an aborting cow is only indicative of exposure to N. caninum and histologic examination of the fetus is necessary for a definitive diagnosis of neosporosis. The brain, heart, liver, placenta, and body fluids or blood serum are the best specimens for diagnosis and diagnostic rates are higher if multiple tissues are examined. Although lesions of neosporosis are found in several organs, fetal brain is the most consistently affected organ. Because most aborted fetuses are likely to be autolyzed, even semi-liquid brain tissue should be fixed in 10% buffered neutral formalin for histologic examination of hematoxylin and eosin (HE) stained sections. Immunohistochemistry is necessary because there are generally only a few N. caninum present in autolyzed tissues and these are often not visible in H and E stained sections. The most characteristic lesion of neosporosis is focal encephalitis characterized by necrosis and nonsuppurative inflammation (Barr et al., 1991). Hepatitis is more common in epizootic than sporadic abortions. Lesions are also present in placenta but protozoa are difficult to find.
The efficiency of the diagnosis by PCR is dependent on the laboratory, stage of the autolysis of the fetus, and sampling procedures. Although immunohistochemical demonstration of N. caninum in lesions is the best evidence for etiology of abortion at the present time, it is very insensitive. Neospora caninum DNA can be detected by PCR in formalin-fixed, paraffin-embedded bovine aborted brain tissue.
Several serologic tests can be used to detect N. caninum antibodies including various ELISAs, the indirect fluorescent antibody test (IFAT), and the Neospora agglutination test (NAT). Immunoblots are useful in detecting N. caninum-specific antibodies. Avidity-ELISAs designed to distinguish recent and chronic infections in cattle appear promising to distinguish endemic and epidemic abortion.
Finding N. caninum antibody in serum from the fetus can establish N. caninum infection, but a negative result is not informative because antibody synthesis in the fetus is dependent on the stage of gestation, level of exposure, and the time between infection and abortion. Even a low IFAT titer of 1:25 should be regarded as specific for N. caninum infection, especially in fetuses. Immunoblotting using N. caninum specific antigen improves diagnosis. Although blood serum or any body fluid from the fetus may be used for serologic diagnosis, peritoneal fluid is better than other body fluids. In calves, presuckling serum can be submitted for diagnosis of congenital infection.
The definitive antibody level that should be considered diagnostic for neosporosis has not been established for bovines because of the uncertainty of serologic diagnosis in chronically infected animals and the availability of sera from noninfected cattle. In serological assays, titer and absorbance values are dependant on antigen composition, secondary antibodies and other reagents. Further, cut-off levels can be arbitrarily selected to provide sensitivity and specificity requested for a particular application. The age and class of an animal may also affect selection of a cut-off level. Although N. caninum is closely related to Toxoplasma gondii, Sarcocystis species, and other apicomplexans, cross reactivity has not been a major issue. Antibody titers in general are higher in cattle that have aborted due to neosporosis than those with normal pregnancy; however, titers in individual cows cannot determine etiology of abortions.
Toxoplasma gondii and Sarcocystis cruzi are 2 other protozoans that should be considered in the differential diagnosis of protozoal abortion in cattle. Immunohistochemical and detection of parasite DNA by PCR can distinguish them from N. caninum. Sarcocystis cruzi forms schizonts in vascular endothelium and is rarely (< 0.1%) found in aborted fetal brains, whereas N. caninum is usually located in extravascular tissues. Additionally, there are no immature schizonts in N. caninum infection in contrast with S. cruzi infections. Infection by T. gondii in bovine fetuses is rare (Canada et al., 2002b).
Attempts at isolation of viable N. caninum (Table 2) by bioassay in mice or cell culture have been largely unsuccessful and little is known of the antigenic variability among isolates of N. caninum, especially isolates from healthy animals. Many attempts to isolate viable N. caninum were unsuccessful because most N. caninum in fetuses die with the host. It is easier to isolate N. caninum from neural tissues of congenitally-infected full term calves (Table 2) because tissue cysts are likely to be present and tissue cysts are relatively more resistant to autolysis than tachyzoites.
Pathogenesis of abortion
Cows with N. caninum antibodies (seropositive) are more likely to abort than seronegative cows. There is a rise in antibody titers 4 to 5 months before parturition. These observations strongly suggest reactivation of latent infection. Little is known of the mechanism of reactivation. It is likely that there is parasitemia during pregnancy leading to fetal infection. However, N. caninum has never been identified in histologic sections of adult cows and there is a single report of isolation of viable N. caninum from the brain of an adult cow (Sawada et al., 2000). Although it is reasonable to speculate that pregnancy-induced immune-suppression may reactivate latent tissue cysts of N. caninum, such a mechanism has not been demonstrated for neosporosis.
Little is known at the present time concerning the pathogenesis of infection in cows following infection with a natural route (oral) and using the naturally transmitted stage of the parasite (oocyst) although oocysts are infective to cattle by the oral route. Results of experiments with dairy or beef cows inoculated parenterally with N. caninum tachyzoites indicate that the fetus can become infected and sometimes diseased by 4 weeks after inoculation of the parasite. Even at the early stage, there were lesions in placenta and the CNS and encephalitis was the predominent lesion. The extent of damage to placenta directly due to multiplication of N. caninum and by immune response is not known (Buxton et al., 2002). Gestational age may determine the outcome of infection. Fetuses infected early in pregnancy are likely to die.
Neospora caninum is a primary pathogen in cattle
Neospora caninum is one of the most efficiently transplacentally-transmitted organisms in cattle. In some herds up to 90% of cattle are infected, and most calves born congenitally-infected with N. caninum remain healthy. Therefore, there is a debate concerning whether N. caninum causes abortion in cattle or whether it is a bystander (Thurmond et al., 1999). All evidence at the present time indicates that N. caninum is a primary pathogen. However, there is a need to distinguish clinical and nonclinical N. caninum infections. In the initial reports by Anderson et al. (1991) and Barr et al. (1991), the following criteria were established to diagnose N. caninum abortion. First, the fetuses had lesions, mostly encephalitis. Second, N. caninum was found in lesions. In my opinion, identification of organisms in lesions is a good evidence for the etiology of the lesions. Additionally, abortion has been induced in experimentally-infected cattle. The percentage of fetuses that have histologically detectable N. caninum and no lesions is unknown. Therefore, detection of N. caninum DNA alone in aborted fetuses may not be enough to establish cause and effect relationship. Host factors (cytokines, hormones) that may contribute to pathogenesis of N. caninum infection have not yet been established (Buxton et al., 2002; Innes et al., 2002).
Transmission
There is no cow to cow transmission of N. caninum. In the study by Anderson et al. (1997), 25 seronegative heifers were housed with 25 seropositive heifers since birth and their progeny were evaluated for N. caninum infection. The seronegative heifers remained seronegative and gave birth to calves not infected with N. caninum. Seropositive heifers remained clinically normal but gave birth to congenitally-infected calves. Seven of these congenitally-infected calves were necropsied; all had histologic evidence of N. caninum infection and 4 were recumbent. Although most N. caninum infections in cattle are transmitted transplacentally, postnatal rates have been variable depending on the region of the country, type of test used and cut-off values used.
It is unlikely that N. caninum is transmitted venereally or by embryo transfer in cattle and embryo transfer is even recommended as a method of control to prevent vertical transmission (Baillargeon et al., 2001). These researchers conducted an important study on embryo transfer and N. caninum infection involving 87 recipient cows or heifers. Neospora caninum infection was not demonstrable in any of the 70 fetuses or calves born to seronegative cows transplanted embryos from seropositive donors whereas 5 of 6 calves resulting from embryo transfer from seronegative donors to seropositive recipients were infected with N. caninum. Landmann et al. (2002) confirmed these findings and showed that commercially used embryo transfer procedures also prevented transfer of N. caninum from seropositive cows to seronegative recipients. Additionally, preimplanted bovine embryos are resistant to N. caninum invasion (Bielanski et al., 2002).
Lactogenic transmission of N. caninum has been demonstrated experimentally in newborn calves fed colostrum spiked with tachyzoites, but there is no evidence that it occurs naturally (Davison et al., 2001); dogs fed milk spiked with N. caninum tachyzoites did not shed oocysts (Dijkstra et al., 2001).
Economic impact
There are no firm data on the economic losses due to neosporosis to cattle industry anywhere in the world but losses are estimated in millions of dollars. As many as 42% of cows may abort due to neosporosis, the economic impact will depend on the direct cost and value of fetuses lost. Indirect costs include professional help and costs associated with establishing diagnosis, rebreeding, possible loss of milk yield, and replacement costs if aborted cows are culled (Thurmond and Hietala, 1996, 1997; Hernandez et al., 2001).
In general, less is known of the causes of abortion in beef cattle than in dairy cattle because of the difficulty of finding small fetuses expelled in the first trimester. Therefore, there are no accurate assessments of Neospora-induced losses in beef cattle. Because clinical disease has not been reported in calves older than 2 months of age, there is no direct evidence of N. caninum-associated morbidity in adult cattle. However, in a sero-epidemiological study, Barling et al. (2000) found a positive association between N. caninum antibody status of the calf and weight gain and projected a $15.62 loss per calf.
Epidemiology and control
As stated earlier, N. caninum is efficiently transmitted vertically in cattle, perhaps for several generations. Therefore, culling is the only way at present to prevent this transmission from cow to heifer. Until the discovery of the resistant stage (oocyst) of N. caninum, horizontal transmission was unexplained. Evidence is accumulating that transmission by oocysts may be more prevalent than initially realized, at least in some parts of the world. Storms of abortions, abortion in recently infected cattle as evidenced by low avidity antibodies, and seroconversion in a high portion of cattle, indicate transmission of N. caninum by fecal-oral route. Postnatal transmission also occurs in herds not experiencing storms of abortions and seroepidemiological data support the role of the dog in the life cycle of N. caninum.
How dogs become infected in nature is not known. Small animals that dogs might prey or consume naturally have not been identified as a natural host for N. caninum. Consumption of aborted bovine fetuses does not appear to be an important source of N. caninum infection in dogs. The consumption of placental membranes may be a source of N. caninum infection in dogs because the parasite has been found in naturally-infected placentas and dogs fed placentas shed N. caninum oocysts. Little is known at present regarding the frequency of shedding of N. caninum oocysts by canids in nature, the resistance of the oocysts, and whether dogs shed oocysts more than once. Until more definitive hosts of N. caninum are found, dogs should not be allowed to eat aborted fetuses, fetal membranes or dead calves. Drugs that will prevent transmission of the parasite for the dam to the fetus are unknown but research is continuing in this area.
There is no proven vaccine to prevent N. caninum abortion in cattle (Innes et al., 2002). However, encouraging results have been obtained in mice vaccinated with killed parasites or their products. Vaccination of mice before pregnancy with killed N. caninum tachyzoites blocked transplacental transfer of N. caninum when mice were challenged during pregnancy demonstrated protective immunity to congenital transfer of N. caninum infection has been demonstrated in experimentally-infected cattle (Innes et al., 2001b). As mentioned earlier, protective immunity appears to develop which reduces or prevents subsequent abortion after a primary infection and resultant abortion in cattle (Anderson et al., 1995; McAllister et al., 2000). This protective immunity appears to be more effective in cows that are infected again from an exogenous source (oocysts) than in cows that relapse from an endogenous infection (Trees and Williams, 2003). Therefore, inducing protective immunity in cows that are already infected naturally is a problem.
NEOSPOROSIS IN DOGS
Neosporosis was first recognized in dogs in Norway by Bjerkås et al. (1984). Retrospectively, the earliest record of neosporosis in dogs was an outbreak in 1957 in the U.S. (Dubey et al., 1990a). Worldwide reports of clinical and subclinical infections were summarized by Dubey and Lindsay (1996) and Lindsay and Dubey (2000) and these references are not repeated here. Since then, antibodies to N. caninum were reported in 121 of 320 (37.8%) dogs from Argentina (Basso et al., 2001b), 22% of 200 dogs from New Zealand (Reichel et al., 1998), 10% of 150 dogs from Turkey (Coškun et al., 2000), 6.7% of 163 dogs from Brazil (Mineo et al., 2001), 10% of 500 pet dogs and 25% of 611 stray dogs from Brazil (Gennari et al., 2002), 6.4% of 1,058 dogs from Italy (Cringoli et al., 2002), and in 12.% of 120 urban and 26% of 81 rural dogs from Chile (Patitucci et al., 2001). Klein and Müller (2001) reported N. caninum antibodies in 4% of 50 dogs in Germany without clinical signs and 13% of 200 dogs with clinical signs. Antibodies to N. caninum were found in 21.6% of 134 dogs from cattle farms in Parana, Brazil (de Souza et al., 2002).
Three surveys compared the prevalence of N. caninum in urban and rural dogs. Sawada et al. (1998) reported N. caninum antibodies in 31% of 48 dogs from dairy farms and 7% of 198 dogs from urban areas in Japan. Wouda et al. (1999b) reported a higher prevalence in farm dogs (23.6% of 152) versus urban dogs (5.5% of 344) from the Netherlands. Basso et al. (2001b) reported higher seroprevalence in dogs from dairy farms (48% of 125) and beef farms (54.2% of 35) than in dogs from urban areas (22.2% of 160) of Argentina.
In addition to clinical infections summarized by Dubey and Lindsay (1996) and Lindsay and Dubey (2000), Boydell and Brogan (2000), Cantile and Arispici (2002) reported clinical neurologic neosporosis in dogs. Four cases of cutaneous neosporosis were recently reported, including mixed infection with Leishmania sp. in 1 dog (Tarantino et al., 2001; Perlé et al., 2001; Ordeix et al., 2002).
Barber and Trees (1998) reported an interesting seroepidemiologic study of N. caninum infections in 373 breeding dogs in U.K. Fifty (13.4%) of 373 bitches had an IFAT titer of > 1:50. Initially, approximately 50% pups born were seropositive and approximately 25% of pups born from seropositive dams developed neosporosis-like illness. Three bitches produced successive litters of N. caninum infected pups. In subsequent breeding only 3 of 118 pups from seronegative dams were seropositive. Thus, vertical transmission alone could not maintain the parasite in dogs (Barber and Trees, 1998).
The most severe cases of neosporosis occur in young, congenitally infected pups. Young dogs develop hind limb paresis that develops into a progressive paralysis. Neurologic signs are dependent on the site parasitized. The hind limbs are more severely affected than the front limbs and often in rigid hyperextention. Other dysfunctions which occur include difficulty in swallowing, paralysis of the jaw, muscle flaccidity, muscle atrophy and even heart failure. Dogs with hind limb paralysis may be alert and survive for months.
The disease may be localized or generalized and virtually all organs may be involved including the skin. Dermatitis may be severe involving enormous numbers of N. caninum. Dogs of any age can be affected. Fatal neosporosis has been reported in eight to 15-year-old dogs.
Subclinically infected bitches can transmit the parasite to their fetuses, and successive litters from the same bitch may be born infected. Whether there is breed predisposition and differential sex susceptibility to neosporosis in dogs is not known. Most described cases have been in Labrador retrievers, Boxers, Greyhounds, Golden retrievers, and Basset hounds.
Neospora caninum has been isolated several times from dogs, mostly from dogs with neuromuscular signs (Table 3). Nothing is known of the isolates of N. caninum from dogs without any clinical signs.
NEOSPOROSIS IN WATER BUFFALOES
Water buffaloes (Bubalus bubalis) are important to the economy of several countries including Brazil, India, Italy, and Vietnam. Prevalence of antibodies indicates that buffaloes in four countries were exposed to N. caninum (Table 4).
Little is known of the role of N. caninum in causing abortion in buffaloes. Guarino et al. (2000) reported lesions and Neospora-like tissue cysts in 2 of 4 fetuses aborted from 2 dairy farms in Italy; this report needs confirmation.
NEOSPOROSIS IN SHEEP
Neospora caninum was first diagnosed in a congenitally-infected lamb in England, (Dubey et al., 1990b). Historically, this was the first record of N. caninum-like infection in a ruminant (Hartley and Bridge, 1975). The lamb was born weak, partially ataxic, and died at one week of age. There was unilateral reduction of gray matter in the ventral horn and local cavitation. This case was published because congenital deformities due to toxoplasmosis had not been seen in any animal (other than humans), only tissue cysts and not tachyzoites were seen. I had known of this case even before it was published. After the discovery of the N. caninum in dogs in 1988, I contacted William Hartley and retrospectively examined tissues of this lamb and an identical case in a calf that was added as an addendum to the case in lamb (Hartley and Bridge, 1975); the calf was from Australia. Subsequently, clinical ovine neosporosis was reported from Japan. Kobayashi et al. (2001) found naturally-infected neosporosis in ewe and her twin fetus in Japan. Focal encephalitis and thick-walled N. caninum tissue cysts were seen in all three animals. Koyama et al. (2001) obtained the first isolate of N. caninum from another adult ewe.
Little is known of the seroprevalence of N. caninum in naturally exposed sheep and evidence for N. caninum as an ovine abortifacient in the field has not been found (Otter et al., 1997). In a recent survey study from U.K., only 3 of 660 ewes that had aborted had antibodies to N. caninum (Helmick et al., 2002). Pregnant sheep, however, are highly susceptible to experimental infection with N. caninum tachyzoites (Dubey and Lindsay, 1990; McAllister et al., 1996; Buxton et al., 1997b, 1998; Jolley et al., 1999; Innes et al., 2001a) and are an alternative ruminant model for bovine neosporosis. Sheep can also be infected orally with N. caninum oocysts (O'Handley et al., 2002).
NEOSPOROSIS IN GOATS
Abortion and neonatal mortality associated with N. caninum was reported in pygmy goats in the U.S. (Barr et al., 1992; Dubey et al., 1992), in a dairy goat herd in Costa Rica (Dubey et al., 1996a), and in a 3-day-old dairy goat from Brazil (Corbellini et al., 2001). Antibodies to N. caninum were found in 5 of 77 dairy goats in Costa Rica that had aborted. Little is known of the seroprevalence of N. caninum antibodies in goats. Ooi et al. (2000) did not find N. caninum antibodies in 24 goats from Taiwan.
Pygmy goats, however, are quite susceptible to experimental N. caninum infection. Goats inoculated N. caninum during pregnancy aborted N. caninum-infected fetuses (Lindsay et al., 1995).
NEOSPOROSIS IN HORSES
Neospora-like parasites have been found in tissues of two aborted foals (Dubey and Porterfield, 1990; Pronost et al., 1999), a congenitally-infected foal (Lindsay et al., 1996b) and five adult horses (Gray et al., 1996; Daft et al., 1996; Marsh et al., 1996; Hamir et al., 1998; Cheadle et al., 1999b). Marsh et al. (1998) proposed a new name, Neospora hughesi, for the parasite in the horse they reported in 1996. Three isolates of N. hughesi from adult horses have been reported (Marsh et al., 1998; Cheadle et al., 1999b; Dubey et al., 2001). Molecular and biological characteristics of these three isolates have been reported (Cheadle et al., 1999a; Walsh et al., 2000; Dubey et al., 2001; Marsh et al., 2001).
Tissue cysts of N. hughesi were smaller than N. caninum with thinner cyst walls (less than 1.0 µm thick), and bradyzoites were smaller than those of N. caninum (Marsh et al., 1998). It is, however, not clear at the present time whether N. hughesi is the sole species of Neospora that infects horses. Thick walled tissue cysts, characteristics of N. caninum were reported from a horse from California (Daft et al., 1996) and a congenitally infected foal from Wisconsin (Lindsay et al., 1996b).
Seroprevalence of Neospora infections in horses is summarized in Table 5. In two surveys or horses in France and the U.S. the seroprevalence by NAT was 23%.
NEOSPORA INFECTIONS IN HUMANS
Because two rhesus monkeys (Macaca mulata) have been successfully infected with N. caninum (Barr et al., 1994), there is a concern about the zoonotic potential of N. caninum. However, at present there is no evidence that N. caninum successfully infects humans. Graham et al. (1999) did not find N. caninum antibodies in 1:160 dilution of sera from 199 blood donors and 48 agricultural workers in Northern Ireland. Petersen et al. (1999) did not find antibodies to N. caninum in 76 women with history of abortion using ELISA, immunoblotting, and IFAT. Low level IFAT (1:100) antibodies were reported by Tranas et al. (1999) in 69 (6.7%) of 1,029 sera from blood donors in California, however, all sera were negative at 1:200 dilution. Nam et al. (1998) reported antibodies to N. caninum in human sera using the immunoblots; however, these sera were negative by NAT. Trees and Williams. (2000) found low (IFAT < 1:200) in 2 of 500 (400 farm workers and 100 women with recurrent abortions) people in England. In conclusion, there is no evidence that N. caninum infection is zoonotic.
NEOSPOROSIS IN WILDLIFE
Neosporosis was diagnosed at necropsy in two black-tailed deer (Odocoileus hemionus columbianus) found dead in the wild in California (Woods et al., 1994), in a captive deer in zoos in France (Dubey et al., 1996b, and Germany (Peters et al., 2001b. Neospora caninum tissue cysts were found in the brain of a full term stillborn deer (Cervus eldi siamensis) in a Paris Zoo (Dubey et al., 1996b. Peters et al. (2001b) reported antibodies to N. caninum in fetal fluid and N. caninum DNA by PCR in the brain, heart, lung, liver and spleen of 2 full term twin calves of antelope (Tragelaphus imberberis) in a zoo in Hannover, Germany.
Neosporosis was reported in a rhinoceros (Ceratotherium simum) calf (Williams et al., 2002). The animal died suddenly, considered to be due to myocarditis. Rhinoceroses are related to equids, all belonging to the order Perissodactyla.
Finding of antibodies in 40% of wild deer (Table 9) indicates there may be a sylvatic cycle of N. caninum. A large percentage of these deer had high titered (> 1:1,600) N. caninum antibodies and the prevalence did not increase with age indicating that congenital transmission (Dubey et al., 1999a). Similarly, red foxes in Belgium had a high seroprevalence of N. caninum. Schares et al. (2001) presented evidence of congenital infection of N. caninum in foxes.