Nosocomial submandibular infections with dipterous fly larvae
Article information
Abstract
In September 1998, a case of nosocomial cutaneous myiasis caused by Lucilia sericata (Meigen, 1826) in a 77-year-old male was found. The patient had been receiving partial maxillectomy due to the presence of malignant tumor on premaxilla. This is the first verified case involving Lucilia sericata in Taegu, Korea. In the present paper, the salient morphological features of the third instar larvae involved have been studied.
INTRODUCTION
Myiasis is well recognized as causing infections in humans and vertebrate animals with dipterous larvae which, at least for a certain period of time, feed on the host's dead or living tissue, liquid body substances, or ingested food (Zumpt, 1965). As noted by Beaver et al. (1984), these Diptera are medically classified into three groups according to the site of the lesions: specific, semispecific and accidental myiasis producing flies. Nosocomial myiasis, although rare, is sometimes reported in debilitated patients, some of which were diabetic (Mielke and Schlote, 1980; Smith and Clevenger, 1986; Burgess or Davies, 1991).
Some of the contributing factors include disturbed consciousness or hypoesthesia that prevent the patient's sensation of fly contact, and paralysis or immobility that prevent from fending off the fly detected (Lettau, 1991; Daniel et al., 1994; Amitay et al., 1998). Recent findings from the United States, Puerto Rico, Canada, Honduras, Israel and others indicate that nosocomial myiasis is probably under-reported.
It is likely that hospital-acquired myiasis is under-reported for a number of reasons and one of the reasons may be related to the institutional risk management decisions. It usually occurs during summer months when fly populations are most dense, and most of the larvae are identified as Phaenicia sericata (Greenberg, 1984), Lucilia sericata (Amitay et al., 1998), Cochiomyia macellaria (Fox and Rodriguez-Torrens, 1972; Smith and Clevenger, 1986; Josephson and Krajden, 1993), and Cochliomyia hominivorax (de Kaminsky RG, 1993), all of which belong to the family Calliphoridae.
We are not aware of any previous reports with respect to opportunistic cutaneous myiasis following radiotherapy for a squamous cell carcinoma on the submandibular area. This article describes a recent case and discusses nosocomial myiasis.
CASE HISTORY
A 77-year-old man with partial maxillectomy due to the presence of metastatic tumor on the premaxilla was admitted to the Dongsan Hospital, Taegu, Korea, from 3 August to 18 September, 1998. After 7 weeks of radiotherapy for a 7 cm-diameter squamous cell carcinoma on the submandibular area with 7000 cGy (Siemens linear accelerator-6MeV photon), he was discharged from the hospital on 18 September, 1998.
The patient reported increasing pain from the lesion 3 days prior to presentation, and ivory-colored larvae were seen in the central portion of the lesion when he was admitted to the Dongsan Hospital in Taegu, Korea, on 20 of September, 1998.
Examination showed a 7 cm-diameter necrotic ulcer, with heaped up borders, in the submandibular area. The lesion was filled by serosanguinous fluid containing 20 fly larvae. He was treated intravenous injection with benzyl penicillin. All the necrotic tissue and the infesting larvae were removed. The larvae were 8 to 11 mm long (Fig. 1). The wound was dressed with 10% povidone iodine and paraffin gauze. A few organisms were fixed in 10% formalin and used for identification. The larva was identified by Dr. Hiromu Kurahashi at Department of Medical Entomology, National Institute of Infections Diseases, Japan, as a third instar larva of Lucilia sericata (Meigen, 1826).
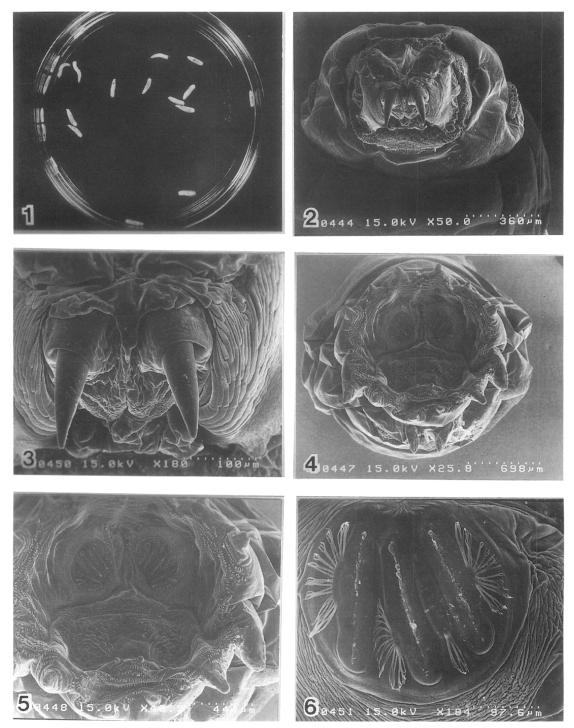
Fig. 1. Twenty larvae of Lucilia sericata removed from the wound. Figs. 2-6. Scanning electron microscopy of the larva. Anterior end with oral hooks (×50). 3. Oral hooks (×180). 4. Caudal end with posterior spiracles. Spiracular area surrounded by 10 tubercles (×25). 5. Posterior spiracles which have three slit-like opening (×50). 6. Posterior spiracles (×180).
For scanning electron microscopic studies, specimens were washed three times in buffer solution, fixed with 3% glutaraldehyde for 2 hours in 0.1 M phosphate buffer solution, pH 7.4, and post-fixed with 1% osmium tetroxide. They were further dehydrated with a graded series of ethylalcohol and iso-amyl acetate, and critical pointed dried. The specimens were coated with gold, using Hitachi E-1030 ion sputtering apparatus, and then examined with a S-4200 scanning electron microscope at an accelerating voltage of 15 kv.
The scanning electron microscopic inspection of larvae is rewarding. The pointed end of the larva is anterior, and the broadly truncate end is posterior. The larva holds on with two sickle-shaped oral hooks on its bulbous head (Fig. 2 and Fig. 3), and it also has rows of concentric, backward-facing, spines and hooks (Fig. 3) which facilitate burrowing and rasping of the host tissues. This explains the sensation of pain as the larva moves around (Gordon et al., 1995). The larva breathes through the posterior spiracles (Fig. 4, Fig. 5 and Fig. 6) which have three slit-like opening. Posterior spiracles with button area well chitinized and ring (peritreme) complete, button area a part of the ring; slits nearly straight; Spiracular area surrounded by 10 tubercles (Fig. 6).
DISCUSSION
Nosocomial infection is defined as the infection that is caused during or after the hospitalization which was neither present nor incubating at the time of admission (Wenzel, 1987; Barrett-Connor et al., 1978). Of the various types of myiasis, only the secondary (accidental, facultative) myiasis is potentially nosocomial. As noted by Greenburg (1984), some of the factors that frequently produce myiasis include (1) helpless and debilitated individuals - helpless individuals can't be a factor causing myiasis ..., (2) blood or odors of decomposition, (3) neglect of nursing or custodial personnel, and (4) summer season. Seventeen reported cases from various countries showed that of nosocomial myiasis were caused by maggots of facultative and obligatory parasitic species of flies, and their characteristics are summarized in Table 1.
In practice, there is no indication of 'true' myiasis in both community-acquired cases as well as nosocomial infestations, because hospital-acquired myiasis is probably under-reported for a number of reasons and some of the cases are squelched by hospital administrators, risk managers, and public relations departments for obvious medicolegal and political reasons. Such considerations were also recognized by Smith and Clevenger (1986), Lettau (1991), Josephson and Krajden (1993), and Daniel et al. (1994). The under-reporting of nosocomial myiasis is further supported by a prospective multihospital study in Brisbane, Australia, that found 14 infestations, of which six were nosocomial, over a period of 17 months with clustering of cases during hot weather (Lukin, 1989). He also recorded that most patients were elderly, with either diabetes or peripheral vascular disease, and had myiasis in foot or ankle wounds. The predisposing conditions included chronic ulcerations of the ankle, burns, gangrene, traumatic laceration, and surgery, including skin grafting. Chigusa et al. (1996) also indicated that patients with psychiatric disorders such as schizophrenia, as well elderly and debilitated persons, should be protected from flies, because of their autism and/or diminished sensitivity, which may make it easy for flies to deposit eggs or larvae on the patient's body surface or orifices.
The reported cases, as shown in Table 1, had similar predisposing features. All occurred during summer months and were hospital-acquired. We are not aware of any previous reports of cutaneous myiasis complicating radiotherapy in Korea. Although opportunistic cutaneous myiasis of the head is rare, a case has been reported complicating the excision of a basal cell carcinoma of the eyelid by Bosniak and Schiller (1990) in the United States, and a case of opportunistic cutaneous myiasis following radiotherapy for a squamous cell carcinoma of the left temple by Phillips and Marsden (1993) in the United Kingdom. On the basis of previously reported data and our own findings, we found, as expected, that all three cases occurred during the summer. This is when the fly population is in its greatest density. Although two previously reported cases involved Calliphorid larvae, our case was due to Lucilia larvae.
As indicated by Lettau (1991), the prevention measures of nosocomial myiasis is directly related to the flies. Sanitary and efficient waste disposals supplemented by some insecticide sprays should minimize fly density. Screens or sealed windows will provide physical barriers. Wound care and dressing, as well as attention to the hygiene of the patients will also decrease the attractions of flies to the patients. These considerations were also made by Josephson and Krajden (1993).
