Determination of antigenic domain in GST fused major surface protein (Nc-p43) of Neospora caninum
Article information
Abstract
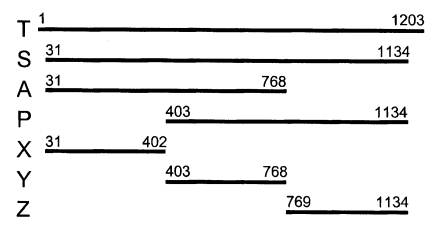
The antigenic domain of the major surface protein (Nc-p43) of Neospora caninum was examined by polymerase chain reaction of its gene fragments and recombinant expression as GST fusion proteins. The fragments of Nc-p43 were as follow: a total open reading frame (OFR), T; OFR without signal sequence and C-terminal hydrophobic sequence, S; N-terminal 2/3 parts of S, A; C-terminal 2/3 parts, P; N-terminal 1/3 part, X; middle 1/3 part, Y; and C-terminal 1/3 part, Z, respectively. The DNA fragments were cloned into pGEX-4T vector. Recombinant plasmids transformed into Escherichia coli of BL21 pLysS (DE3) strain were induced to express GST or GST fused fragments of Nc-p43 such as 69 kDa protein for T, 66 kDa for S, 52 kDa for A, 53 kDa for P, and 40 kDa proteins for X, Y, and Z, respectively in SDS-PAGE. The Nc-p43 fragments of T, S, and P reacted with a bovine serum of neosporosis while those of A, X, Y, and Z together with GST did not in the western blot. These findings suggest that the antigenic domain of Nc-p43 of N. caninum may be localized in the C-terminal 2/3 parts. Together with A19 clone in SAG1 of Toxoplasma gondii (Nam et al., 1996), the P fragment of Nc-p43 could be used as efficient antigens to diagnose and differentiate those infections with both species.
INTRODUCTION
Neospora caninum is an apicomplexan parasite that was originally identified as an aetiologic agent of neurological disease in dogs and has been known to be associated with abortions in cattle (Dubey et al., 1988; Dubey and Lindsay, 1993). Both tachyzoites and cyst-forming bradyzoites have been characterized in the asexual phase of N. caninum (Lindsay et al., 1993). And recently, it was described that dogs are definitive hosts of this parasite (McAllister et al., 1998).
The surface proteins of apicomplexan parasites are often immunodominant and seem to be of particular interest as a diagnostic tool and/or as vaccine antigens (Bulow and Boothroyd, 1991; Lunden et al., 1997). Many surface proteins of N. caninum have been identified including Nc-p43 (Hemphill and Gottstein, 1996), p29 and p35 (Howe et al., 1998), and p38 (Schares et al., 2000). Their functions have not been elucidated, however, there is an indirect evidence that at least one of these antigens, Nc-p43, is involved in the attachment of the parasite to host cells (Hemphill, 1996). A complete Nc-p43 gene has been cloned for the DNA vaccine trials (Nishikawa et al., 2000, 2001). Therefore, the essential antigenic determinants should be investigated for cloning in order to produce a large quantity of antigens in recombinant forms for the diagnostic purpose. And for the protein or DNA vaccine, cloning of crucial epitope should be performed to eliminate the unnecessary interaction between processed antigen of non-essential and host immune machinery. In this study, fragments of Nc-p43 were expressed as GST fusion proteins to examine the antigenic domains.
MATERIALS AND METHODS
Parasite
N. caninum tachyzoites of the Nc-1 strain and the Korean isolate (KBA-2, Kim et al., 2000) were maintained in Vero cells (CRL 6318, ATCC, Rockville, MD) in DMEM supplemented with 10% FBS (Gibco BRL Co., Rockville, MD). Pure N. caninum tachyzoites were obtained from the supernatant of 3 to 4 days confluent culture.
Reverse transcriptase-polymerase chain reaction (RT-PCR) and amplification of Nc-p43 gene fragments
Total RNA was purified from the tachyzoite extracts with Tri reagent (Sigma Chem. Co., St. Louis, MO) and used as templates. RT-PCR was performed with a primer set of T in Table 1, which amplified the open reading frame of the major surface membrane antigen (Nc-p43) (GeneBank accession number U93870). Amplified DNAs were cloned into a pGEM-T Easy vector (Promega Corp., Madison, WI) and sequenced with T7 and SP6 primers. Oligonucleotide primers were synthesized according to the coding sequence of GeneBank accession number U93870 as designed in Fig. 1. The DNA sequences of the synthesized primers are listed in Table 1. PCR was done on a subcloned pGEM-T vector with Nc-p43 gene from KBA-2 strain.
Construction of recombinant plasmids and expression of fusion proteins
The amplified DNAs were inserted into pGEM-T Easy vector, and then subcloned into pGEX-4T vector (Amersham Pharmacia Biotech., Uppsala, Sweden) with Eco RI (Gibco BRL) digestion. After confirmning the correct orientation of an insert, plasmids were transformed into Escherichia coli of BL21 pLysS (DE3) strain (Invitrogen, Carolsbad, CA). E. coli cells of log phase were treated with 0.5 mM isopropyl β-D-thiogalactoside (IPTG) for 3 hr at 30℃ to induce the expression of the fusion proteins.
Western blot
Western blot was performed by the method of Towbin et al. (1979). The cell extracts were separated on 12% SDS-PAGE gels and transferred onto nitrocellulose sheets (NC, Schlleicher and Shuell, Keene, NH). The fusion proteins were confirmed with GST detection kit (Amersham Pharmacia Biotech). For the determination of the antigenic domain, NC paper was blocked by 10% rabbit serum in PBS/0.05% Tween-20 overnight. NC paper was then incubated with 1:500 diluted bovine serum of neosporosis (Bae et al., 2000) followed by incubation with 1:5,000 diluted HRP-conjugated goat anti-bovine IgG antibody (Sigma). They were soaked in enhanced chemiluminescence (ECL) solution (Intron, Daejon, Korea) for 1 min and exposed to an X-ray film (Konica, Tokyo, Japan).
RESULTS
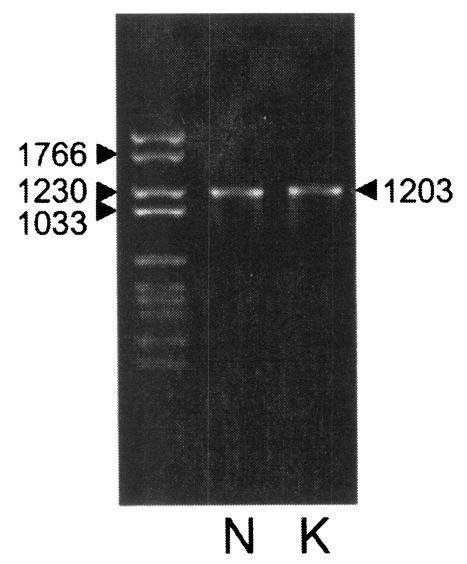
With a set of primers used to amplify the ORF of Nc-p43 gene, a 1,203 bp DNA fragment was obtained by RT-PCR from each Nc-1 and KBA-2 strain of N. caninum (Fig. 2). Each DNA was subcloned into a pGEM-T easy vector for sequencing. The DNA sequencing data of the Nc-p43 gene of the KBA-2 strain revealed a point mutation at the 625th position where cytosine was in place of guanine in the sequence of Nc-1. This affected the deduced amino acid sequence of the 209th residue, which was changed to glutamine (Q) from glutamic acid (E) as shown in Fig. 3. When using the Nc-p43 (T) as a template, a number of Nc-p43 fragments was obtained with sets of primers listed in Table 1. The sizes of these fragments were 1,104 bp for S, 738 bp for A, 732 bp for P, 372 bp for X, 366 bp for Y, and 366 bp for Z as designated in Fig. 1 (Fig. 4). Each DNA was inserted into a pGEM-T Easy vector and then subcloned into a pGEX-4T with Eco RI digestion (data not shown).

Sequence variation in Nc-p43 gene and deduced amino acid of Nc-1 and KBA-2 strains. Point mutation in DNA sequence and deduced amino acid sequence are italicized.

PCR-amplified Nc-p43 coding gene fragments of KBA-2 strain separated in 1.2% agarose gel. Numerals indicate bp.
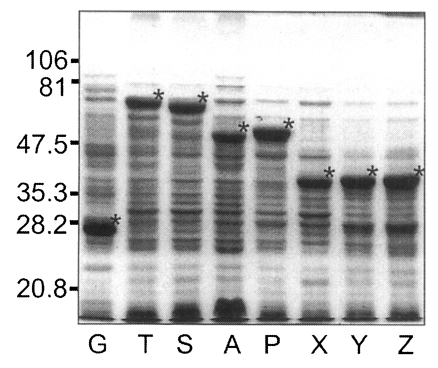
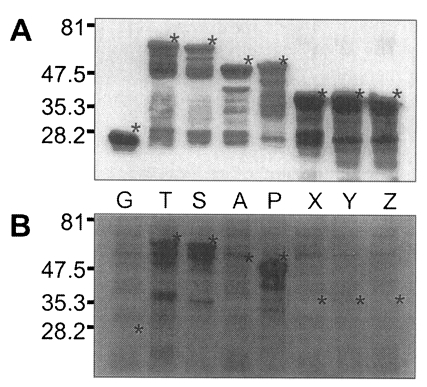
pGEX-4T and recombinant plasmids transformed into E. coli of BL21 pLysS (DE3) strain were induced to express GST or GST fused fragments of Nc-p43 with the addition of IPTG. As shown in Fig. 5, GST was expressed as a 26 kDa protein. GST fusion proteins were expressed as 69 kDa protein for T, 66 kDa for S, 52 kDa for A, 53 kDa for P, and 40 kDa proteins for X, Y, and Z, respectively, when visualized on SDS-PAGE. Each fusion protein was confirmed with GST detection kit (Fig. 6A) as comparable molecular weight with that of SDS-PAGE. In the western blot, Nc-p43 fragments of T, S, and P reacted with a bovine serum of neosporosis while those of A, X, Y, and Z together with GST did not as shown in Fig. 6B.
DISCUSSION
In the western blot, bovine serum of neosporosis detected a P fragment without binding to the subfragments of it, Y nor Z. This suggests that the antigenic domain of Nc-p43 of N. caninum may be localized in the C-terminal 2/3 parts, of which polypeptides of the second 1/3 with the presence of the third 1/3 or the third 1/3 with the presence of the second 1/3 or in the oligopeptides between the margins of the second and the third 1/3 parts. This is comparable to the antigenic domain of the major surface antigen of Toxoplasma gondii, SAG1, in the N-terminal 1/3 with the presence of middle 1/3 (Nam et al., 1996). Although oligopeptides of about 20 amino acids are sufficient to induce B- and T-cell immune responses (Godard et al., 1994), a conformational aid by the other parts seems to be essential to express antigenicity in western blot.
The Korean isolate KBA-2 has a point mutation in the coding gene of Nc-p43, which affects the deduced amino acid at 209th residue from glutamic acid of Nc-1 strain to glutamine. A substitution of glutamic acid, which has an acidic side chain, to an uncharged glutamine residue may contribute to the change in conformation of the antigen. This change in amino acids occurs in the antigenic domain of C-terminal 2/3 of Nc-p43 protein. The extracts of KBA-2 strain was more suitable as a diagnostic antigen than those of Nc-1 for N. caninum infection in Korea (Bae et al., 2000), though there were no differences in morphology of the parasites and pathologic findings in the hosts by the infections (Kim et al., 2000). This partially reflects the prevalent strain of KBA-2 in Korea.
Nc-p43 has been known to play an important role during the initial physical interaction between the parasite and the host cell surface (Hemphill and Gottstein, 1996). And the affinity-purified anti-Nc-p43 antibodies inhibited adhesion and invasion of N. caninum tachyzoites into the host cells (Hemphill, 1996). But the inhibitory effect may result from the aggregation of tachyzoites themselves by the antibody, which merely delay the entry of tachyzoites. Controversially, it is also possible for multiple entry of the tachyzoites of N. caninum into host cells through the crosslinking by the antibody as in the case of Plasmodium falciparum (Ramasamy et al., 2001). And the surface antigens are inserted into the membrane by glycosylphsphatidylinositol (GPI)-anchor (Howe et al., 1998) to evade the host immune by shaking off the attack of the antibody.
Prior to 1988, N. caninum was misdiagnosed as T. gondii due to its close structural similarity (Dubey, 1992). However, due to the ultrasturctural differences (Lindsay et al., 1993) and difference in genetic level (Marsh et al., 1995), it has been possible to distinguish these two species from each other. Although the gene encoding Nc-p43 revealed a sequence homology to the SRS-2 gene of T. gondii (Hemphill et al., 1997; Howe et al., 1998), cross reactivity of this antigen with T. gondii infection seem to be negligible as determined by immunoblotting (Bjerkas et al., 1994; Pare et al., 1997; Bae et al., 2000). Together with A19 in SAG1 of T. gondii (Nam et al., 1996), the P fragment of Nc-p43 could be used as antigens for the diagnosis of N. canimum infection, and furthermore, the antigens could be used to efficiently diffrentiate N. canimum from T. gondii.
Notes
This work was financially supported by the Ministry of Agriculture and Forestry (399002-3) of the Republic of Korea.