Experimental induction of the two-host life cycle of Sarcocystis cruzi between dogs and Korean native calves
Article information
Abstract
Eight dogs were experimentally infected with Sarcocystis by oral inoculation of cardiac muscle from naturally infected cattle. The infected dogs commenced discharging of sporocysts in the feces after 10 to 12 days of inoculation, and continued until 20 and 35 days after inoculation. Three dogs were reinfected with cardiac muscle from the naturally infected cattle. Sporocysts reappeared in the feces on 12 to 13 days after reinfection. Sarcosystis sporocysts collected from the experimentally infected dogs were fed to each of the two 30-day-old Korean native calves. The infected calves remained clinically normal, except for the high fever (≥ 40℃) and decreased hematocrit values on day 30 to 40 post inoculation. Muscular cysts of Sarcocystis were found from infected calves on day 40 post inoculation. Proliferative forms of Sarcocystis were also observed in the muscle of infected calves. These results suggest that the Sarcocystis cruzi found in Korean native cattle has a 2-host life cycle with dogs as the definitive host and Korean native calves as the intermediate host.
INTRODUCTION
Sarcocystis spp. have two-host life cycle in which herbivores act as intermediate hosts that are infected after ingesting sporocysts or oocysts in the feces of the definitive hosts, which in turn acquire the infection by ingesting sarcocysts in the tissues of intermediate hosts (Dubey, 1976; Fayer, 1977; Dubey et al., 1982; Saito and Itagaki 1994). The parasite is not only pathogenic for calves but can cause abortion, clinical illness, and even death in experimentally infected cattle (Johnson et al., 1975; Hong et al., 1982; Frelier and Lewis, 1984). Bovine sarcocystosis is caused by S. cruzi and is known to cause considerable morbidity and mortality in cattle (Dubey, 1976; Frelier and Lewis, 1984; Carrigan, 1986). Surveys of Sarcocystis infection among slaughtered cattle in Korea indicated that the infection rate ranged from 29.1 to 43.6%, and a higher infection rate was observed from older groups of cattle (Kang et al., 1988; Jang et al., 1990; Yang et al., 1990; Park et al., 1994). Even though Sarcocystis infection has been established in experimental dogs fed with bovine cardiac muscle (Park et al., 1994), there have been no attempts to establish muscle cyst in cattle through reinfection of sarcocysts isolated from the infected dogs in Korea. The objective of this investigation was to identify domestic dogs as the definitive hosts of Sarcocystis in Korea, and to demonstrate the life cycle of Sarcocystis through re-infection of cattle with sarcocysts excreted through the feces of the definitive host.
MATERIALS AND METHODS
Preparation of cardiac muscle
Samples of cardiac muscle (about 50 grams) were collected from cattle slaughtered in abattoirs in Seoul, Korea. Most of the cattle included in the study were Korean native cattle, 18-24 months of age. To collect sarcocysts from the muscle, 5 grams from each muscle sample were subjected to 1% pepsin-HCl digestion for 1 hour at 40℃ by the method previously described (Seneviratna et al., 1975) with a slight modification. The digested muscle fluid was sieved and the filtrate centrifuged at 500 g for 5 min. The supernatant was then removed, and the sediment was resuspended in a small volume of saline. Samples were then examined under the light microscope for Sarcocystis bradizoites at 400 × magnification (Kang et al., 1988; Jang et al., 1990).
Experimental animals
Eight dogs and four cats used in this study were raised in a Sarcocystis-free facility. All animals were under 6 months of age and were of mixed breeds. Animals had never been exposed to Sarcocystis, and they were only fed with dry food sold commercially. Eight dogs and two cats were fed with 300 grams of cardiac muscle from the infected cattle. Infected dogs and cats were housed individually from here on. Three Korean native calves raised in the facility of National Veterinary Research and Quarantine Service were used in this study. Two 30-day-old calves (calf 1 and calf 2) were inoculated with 3.0 × 105 and 2.0 × 105 sporocysts of S. cruzi (sometimes oocysts contained) derived from experimentally infected dogs, respectively. A 35-day-old calf (calf 3) served as negative control. The calves were housed individually.
Sporocysts detection in dogs and cats
The feces of dogs and cats were collected and examined daily for the presence of Sarcocystis using the Sheather's sugar floatation method, employing a sucrose of 1.21 specific gravity. The number of sporocysts per gram of feces (SPG) was determined by an EPG counter (FHK, New McMaster). Three dogs of the 8 infected dogs were fed with 300 grams of cardiac muscle from an infected cattle on 50 days after the first infection and the feces of these dogs were examined for sporocysts everyday.
Observation of clinical signs and detection of Sarcocystis bradyzoites in calves
Clinical signs were observed from each calf daily. Also body temperature and packed cell volume (PCV) were recorded from each calf every 5 day intervals until 40 days after the initial inoculation with sporocysts of Sarcocystis. All calves euthanatized at day 40 after inoculation. And proliferative forms of Sarcocystis bradyzoites were detected in 31 muscle samples.
RESULTS
All dogs fed with Sarcosystis-infected cardiac muscle of cattle produced oocysts or sporocysts in their feces. The presence of Sarcocystis in the feces of the two uninfected control dogs and four infected cats was not detected. The infected dogs commenced sporocysts (sometimes oocysts) in the feces from day 10 to day 12 post inoculation (p.i.) and continued during 20 to 35 days. The number of discharged sporocysts peaked on day 20 to 25 p.i. However, two dogs (No. 2 and 11) discharged sporocysts invisibly (Fig. 1). When three of the eight infected dogs were again fed with Sarcosystis-infected cardiac muscle 50 days after the first infection, they began to discharge sporocysts in the feces on day 12 to 13, and continued during 20 days with respect to the second feeding of infected cardiac muscle. The number of sporocysts discharged peaked on day 18 to 24 p.i. (Fig. 2).

Patterns of sporocyst excretion in the feces of dogs inoculated with 300 gm of cardiac muscle of cattle infected with Sarcocystis cruzi.

Patterns of sporocyst excretion of three dogs fed with 300 gm of cardiac muscle from infected cattle on 50 days after first infection.
During the experiment, all of the calves remained clinically healthy and retained normal appetites. The rectal temperature of calf 1 became elevated on day 30 p.i. and persisted until euthanatized, but the rectal temperature of calf 2 was similar to that of negative control (Fig. 3). Packed cell volume (PCV) levels of calf 1 and calf 2 began to decrease on 30 day p.i. (Fig. 4). Muscular cysts were found from both calves which were euthanatized on day 40 p.i. Proliferative forms of sarcocysts were detected in 9 of 31 muscle samples collected of which high concentrations were found in the heart muscle, esophagus, and muscle of thoracic limb (Table 1).
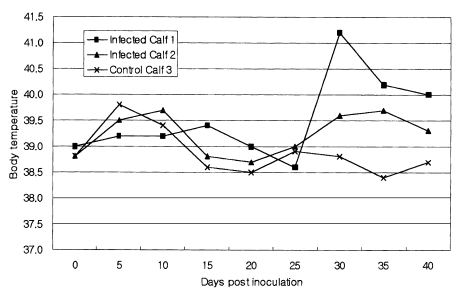
Rectal temperature of Korean native calves experimentally infected with Sarcocystis cruzi sporocysts isolated from experimentally infected dogs.

Patterns of packed cell volume of Korean native calves experimentally infected with Sarcocystis cruzi sporocysts isolated from experimentally infected dogs.
DISCUSSION
S. cruzi has been identified as the most common sarcocysts around the world, and the cattle is known to serve as the intermediate host (Fayer, 1977; Carrigan, 1986; Odening et al., 1995). In Korea, the identification of S. cruzi was reported by Park et al. (1994), who demonstrated that dogs were successfully infected after feeding of cardiac muscle of slaughtered cattle originated from Kangwon-do. The results of this study agreed with previous studies that sporocysts were observed only in dogs experimentally fed with cardiac muscle from infected cattle with sarcocysts and sporocysts appeared in the feces on day 12 to 13 p.i. with a peaked production of sporocysts during day 20 to 25 p.i. We further demonstrated the encystations of sarcocysts in the muscle of cattle by infecting cattle with sporocysts collected from the feces of infected dogs. Advanced initiation of sporocyst production on day 1 to 2 p.i. with a peak production of sporocysts on 18 to 24 days after reinfection in the three reinfected dogs indicate the fact that the immunity against S. cruzi was not fully established in the infected dogs.
The previous reports indicated that the infection rate of Sarcocystis among Korean cattle was as high as 29.1-43.6% (Kang et al., 1988; Jang et al., 1990; Yang et al., 1990), which suggested the existence of the associated life cycle of S. cruzi between domestic cattle and dogs. However, there has not been any reported case of naturally-acquired S. cruzi infection in domestic dogs in Korea, although several investigators have attempted to establish such findings. This study may indicate that naturally occurring infected cases are hard to detect since low number of sporocysts were detected even in the experimentally infected dogs. It is nearly impossible to detect sporocysts from the feces without a concentration method with samples collected during the peak secretion period.
Naturally and experimentally infected cattle with S. cruzi became severely ill or some even died with abortion and lesions similar to those in dalmeny disease (Fayer et al., 1976; Frelier and Lewis, 1984). Furthermore, acute anemia developed which primarily was the result of acute extravascular hemolysis. The most severe pathologic change occurred during day 26 and 33 p.i. (Johnson et al., 1975). In this report, the fluctuation of rectal temperatures and PCV levels were observed, which supported the findings described by above mentioned groups. Our results also suggest that more attention should be given to the pathogenicity of cattle in the field. In conclusion, these results confirmed that the S. cruzi found in Korean native cattle has a 2-host life cycle, with dogs as the definitive host and Korean native calves as the intermediate host.
