Experimental infection of Anopheles sinensis with Korean isolates of Plasmodium vivax
Article information
Abstract
The objectives of the present study were to (1) determine the susceptibility of Anopheles sinensis to Korean isolates of Plasmodium vivax, (2) establish a method to collect large quantities of P. vivax sporozoites for use as antigen in seroepidemiological studies, and (3) investigate the characteristics of Korean isolates of P. vivax sporozoites. Females of Anopheles sinensis were collected at non-epidemic area, Seokwha-ri, Cheongwon-gun and Chungcheongbuk-do using tent-trap methods coupled with dry ice. The females were artificially infected with gametocytes of P. vivax using blood obtained from P. vivax malaria patients. Individual mosquitoes were infected using either a parafilm-covered glass feeding apparatus or were allowed to feed on naturally infected volunteers. Mosquitoes were sacrificed between 16 and 18 days post-feeding and an enzyme-linked immunosorbent assay (ELISA) was used to detect sporozoites. Four (33.4%) of 12 mosquitoes, which were fed on naturally infected volunteers directly, were positive for sporozoites. In cases, the mosquitoes allowed to feed on whole blood which were extract from three different patients with heparin treated vacuutainers using a parafilm-covered glass apparatus. Two of 55 (3.6%) were positive which blood sample was maintained at room temperature for 8 hours, 1 of 68 (1.5%) was positive which blood was maintained at 4℃ for 24 hours and 1 of 47 (2.3%) was positive at 4℃ for 48 hours. The mean number of sporozoites was estimated about 818 (n=8; range of 648-1,056) based on optical density values of ELISA.
INTRODUCTION
Among the many tropical diseases, malaria is the most prevalent worldwide and remains a major health problem. Plasmodium vivax causes the most widespread form of non-falciparum malaria that is infective to humans. It is responsible for significant levels of morbidity that impede the economic development of non-African endemic regions (Galinski and Barnwell, 1996). In Korea, vivax-malaria has been prevalent for many years. However, as the result of national malaria eradication program, in conjunction with assistance from World Health Organization (WHO), the incidence of vivax-malaria has rapidly decreased in Korea (National Malaria Eradication Service, 1966; Paik et al., 1988). Vivax-malaria was believed on eradication from Korea since the late 1970s, although two sporadic cases were reported in the 1980s (Soh et al., 1985).
In 1993, a case of vivax-malaria was reported among Korean soldiers serving in the northern Gyeonggi-do province (Chai et al., 1994). Cho et al. (1994) also reported two cases of civilians infected with P. vivax. Between 1994 and 2000, more than 13,000 patients infected with vivax-malaria were detected near the demilitarized zone (DMZ), which centers on Paju-si, Yeoncheon-gun, Cheorwon-gun, Kimpo-si, Gangwha-gun, Goyang-si, and Dongducheon-si (Lee et al., 1998; Park et al., 2000).
There are several recent studies that have focused on the re-emergence of vivax-malaria in Korea (Chai, 1997, 1999; Kim et al., 1997; Kim et al., 1998; Ree, 1998, 2000; Shim and Kim, 1999). Many of these studies have concentrated on the mosquito vector and have examined several factors including the seasonal prevalence. The use of the Poly-merase Chain Reaction (PCR) for rapid detection of parasites in mosquitoes was also reported (Lee et al., 2000). There were two reports about the infectivities of Anopheles sinensis and An. yatsushiroensis (Ree et al., 1967; Hong, 1977). Ree et al. (1967) discovered 3 sporozoites infected female mosquitoes among 7 An. sinensis which were fed with blood of malaria patient directly. And one female An. sinensis among 7,515 was detected as sporozoites infection in field studies. Hong (1977) also reported 2 sporozoites positive cases among 4,018 females An. sinensis and one oocyst positive case out of 89 female An. yatsushiroensis naturally. In addition to these, he performed artificial infection experiments with An. sinensis and An. yatsushiroensis. As the results of these, one An. sinesis was infected with oocyst among eight tested, three An. yatsushiroensis were infected one with oocysts and two sporozoites among nine tested females. These findings described above were based on dissection method. Dissection method is laborious and time-consuming procedure.
In present study, the enzyme-linked immunosorbent assay (ELISA) was used to detect and calculate the sporozoites infected mosquitoes and their number of sporozoites. This method was developed by Wirtz et al. (1985) for P. vivax sporozoites especially. Monoclonal antibodies used in ELISA was produced against malaria sporozoites antigens, especially repeating epitopes of circumsporozoites (Arnot et al., 1985). Therefore it was well known that sporozoites ELISA method has a high sensitivity, species-specific and specimens can be held in storage (dry or frozen) before processing. The ob-jectives of the present study were to (1) determine the number of sporozoites which develop in the salivary glands of An. sinensis, the main vector of malaria in Korea, and (2) determine the susceptibility of An. sinensis to infected blood under in vitro conditions.
MATERIALS AND METHODS
Collection of mosquito
In Korea, naive female mosquitoes could not be obtained, as this species is not reared under artificial conditions. Therefore, females of An. sinensis were collected at the Seokwha-ri, Cheongwon-gun, Chungcheongbuk-do where is non-epidemic areas of malaria in Korea. Mosquitoes were collected by tent night-biting collections (Beier et al., 1990). Collections were started at 19:00 and continued until 07:00 the next morning.
Source of infected blood
Blood samples were collected 5ߢ by syringe aseptically from different patients infected with P. vivax who had never been abroad. To determine the developmental stage of the parasite within each patient, thin blood films were prepared and examined just before application for bloodmeal. Whole blood samples were stored in refrigerator (4℃) or at room temperature until the mosquitoes were ready to feed. Once the existences of gametocytes were confirmed, the parasitemia was calculated for each of the samples.
Blood feeding
Captured mosquitoes were starved for one day before feeding. To accommodate egg lying, blood-fed mosquitoes were transferred to paper cups filled with approximately 10 mm of water and equipped with a wire net to prevent mosquitoes from sinking. Dead mosquitoes were removed daily. Mosquitoes were fed using two different methods: (1) in vitro feeding and (2) direct feeding. For the direct feeding, mosquitoes were exposed for 20 min. to the forearms of an infected adult P. vivax gametocytemic volunteer who was working in our laboratory (experiment 1, Table 2). With the in vitro feeding, mosquitoes were allowed to engorge on blood collected from P. vivax infected patients through a parafilm covered glass apparatus. The blood was stored at room temperature for 8 hours (experiment 2, Table 2), or 4℃ for 24 hours (experiment 3, Table 2), or 4℃ for 48 hours (experiment 4, Table 2). After feeding was completed, un-engorged mosquitoes were discarded. The fed mos-quitoes were maintained at 22 ± 2℃ with 75 ± 12% relative humidity. Mosquitoes were provided with cotton wool pads soaked in 10% sucrose and were housed with an equatorial photo period (Rosenberg and Rungsiwongse, 1991).
Enzyme-linked immunosorbent assay (ELISA) to detect sporozoites
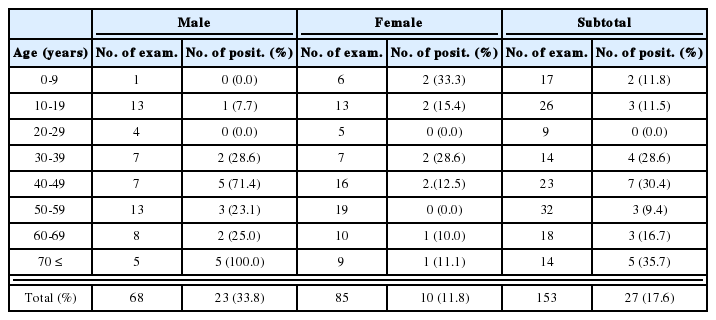
All An. sinensis females survived were killed between 16 and 18 days after feeding a bloodmeal. Mosquitoes were killed by freezing and samples were stored at -20℃ until use. To prepare the samples for ELISA, individual mosquito was placed in 200 µl of blocking buffer (1% bovine serum albumin, 0.5% casein, 0.01% thimerosal, and 0.002% phenol red dissolved in 0.01M phosphate-buffered saline, pH 7.4, 0.5% Nonidet P-40) and homogenized using a pestle. Aedes vexans was used as a negative control in all assays.
In the present study, a two-site homologous sandwich ELISA (Wirtz et al., 1985) was used to calculate the intensity of sporozoites. Dr. Wirtz gave us all reagents including monoclonal antibodies enough kindly. All assays were conducted at room temperature and plates were covered during incubations to prevent evaporation. The wells of disposable polyvinyl chloride, U-bottom microtiter plates (Dynatech) were coated with 50 µl volumes monoclonal antibody P. vivax NSV3 (0.5 µg/ml PBS), covered, and held overnight. Coated wells were aspirated dry, blocked with blocking buffer for 1 hour, the contents aspirated, and mosquito samples were added to the appropriate wells. After 2 hours, the contents of the wells were aspirated, washed three times with PBS-0.05% Tween 20, and 50 µl of the homologous peroxidase-conjugated monoclonal antibody (2 µg/ml in blocking buffer) was added. After 1 hour the contents of the wells were aspirated, washed three times with PBS-Tween 20, and 100 µl of the peroxidase substrate (Kirkegaard & Perry Laboratories) was added to each well. The optical density values (405 nm) were recorded after half an hour using an ELISA plate reader (Dynatech). The positive control of P. vivax-210 type consisted of two Gly-Asp-Arg-Ala-Asp-Gly-Gln-Pro-Ala repeats glutaraldehyde conjugated to BSA (Arnot et al., 1985). These positive synthetic peptides were also reacted in wells, A12 (40 pg), B12 (20 pg), C12 (10 pg), D12 (5 pg), and E12 (2.5 pg), respectively.
Estimation of the number of sporozoites
After measuring the optical density values, the standard curves of each plate were made and evaluate each reaction. Samples with an optical density value of 2 X the mean of the negative controls were considered positive. We calculated the number of sporozoites in parasitized mosquitoes according to the studies by Wirtz et al. (1987). Control equivalences were determined to be 40 pg of synthetic peptide = 400 sporozoites for P. vivax-210 type.
RESULTS
Paraisites of blood sources
The blood sources and their characters were described in Table 1. Blood samples were collected 4 different areas, Paju-si, Yeoncheon-gun, Dongducheon-si and Gangwha-gun. The parasitemia of each sample was counted variously from 640 to 6,640. The number of trophozoites was counted as relatively higher than other stage of parasites. In two of four blood samples, we could not detect any schizonts. But all of them included the male and female gametocytes.
Survival rate of mosquitoes
In the experiment 1, mosquitoes were allowed to feed directly from a volunteer that had detectable gametocytes (one of the authors). A total of 18 An. sinensis were fed to satiety and 12 of these (66.7%) survived. In the experiment 2, a total of 87 An. sinensis fed to satiety and of these, 55 (63.2%) survived for 18 days. In the experiment 3, 68 (90.7%) of the 75 An. sinensis which fed to satiety survived for 16 days. In the experiment 4, 47 (79.7%) of 59 An. sinensis which fed survived for 17 days (Table 2).
Percentage of positive mosquitoes
In the experiment 1, 4 (33.4%) of 12 mosquitoes were positive. In contrast, only 4 (2.4%) of 170 survived An. sinensis fed using the parafilm-covered apparatus were positive for gametocytes. The positive rate for sporozoites was 3.6% (2 of 55) in the experiment 2, 1.5% (1 of 68) in the experiment 3, and 2.1% (1 of 47) in the experiment 4, giving the total average of 4.4% (8/182).
Sporozoite rates
To evaluate the ELISA reactions, we made standard curve with various concentration of circumsporozoite synthetic peptide (Fig. 1). Optical density values of A. vexans (negative control) and uninfected and infected An. sinensis were shown in Fig. 2. Techniques for sporozoite quantification by ELISA have developed; optical density values of samples were compared to standard curves of synthetic peptide and counted sporozoites. Using the technique described by Wirtz et al.(1987), we estimated the number of sporozoites of 8 positive mosquitoes. In the experiment 1, the number of sporozoites for four positive mosquitoes (based on ELISA values) was calculated as 660, 656, 900, and 648, respectively. In the experiment 2, two mosquitoes had sporozoites with numbers of 716 and 928. In the experiment 3, the only one infected mosquito had 1,056 sporozoites. In the experiment 4, the number of sporozoites for the infected mosquito was 976. In total, the mean number of sporozoites for positive mosquitoes (n=8) was 818 (range 648-1,056).
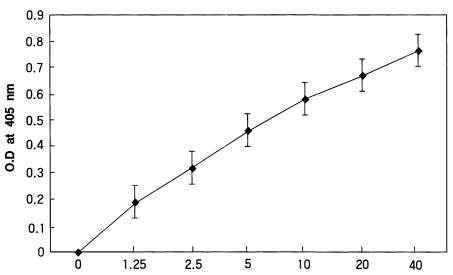
Standard curve of Plasmodium vivax synthetic peptide in the ELISA. (Ordinate = Optical density value at 405 nm)
DISCUSSION
The direct dissection method (Ree et al., 1967; Hong 1977) and polymerase chain reaction (PCR) (Lee et al., 2000) are two methods that have been used to study the main vector of malaria transmission in Korea. These studies have demonstrated that An. sinensis is the main vector responsible for malaria transmission, but An. yatsusiroensis is also a vector (Hong, 1977).
In present study, we attempted to establish how many An. sinensis became infected when fed a gametocytemic bloodmeal under in vitro conditions. An understanding of the susceptibility of this vector will help in estimating how many infected patients will appear, based on the number of positive mosquitoes (i.e., detected by various methods). The ability to cultivate the parasite in vitro will help to produce large numbers of sporozoites that can be used for many things such as genetics, physiological studies and vaccine development.
The data collected in the present study does not necessarily reflect what would occur in the natural situation for several reasons. To perform in vitro infections of An. sinensis with Korean isolates of P. vivax, the mosquitoes were collected in the field. This is because An. sinensis could not be reared under laboratory conditions at the time the present study was undertaken. Therefore, to ensure collection of non-infected mosquitoes, An. sinensis collected in non-epidemic areas, which were more than 150 km from epidemic areas. Furthermore, P. vivax could not maintained under in vitro conditions until present. Therefore, blood samples were collected from patients and used as a source of infection. Despite these difficulties, the present study demonstrated that An. sinensis could be infected with P. vivax through artificial feeding. A total of 239 An. sinensis were fed successfully with gametocytemic blood and, 8 An. sinensis produced sporozoites (4.4%) among 182 survived mosquitoes. The survival rates were revealed differently in each experiment from 63.2% to 90.7%. It might be originated by different longevities of collected mosquitoes in filed. It could be improved their survival rates, if the mosquitoes which are reared in laboratory by artificially were used in experiments. Positive rate (33.4%) of direct feeding case (experiment 1, Table 2) was shown higher infectivity than artificial infections with parafilm covered feeding apparatus. The positive rate was similar or higher than the results obtained by Ree et al. (42.8%, 1967) and Hong (12.5%, 1977). The positive rates of indirect artificial feeding cases (experiment 2, 3 and 4, Table 2) were very low from 1.5% to 3.6%. These results might be caused by number of living gametocytes and their numbers. That is, positive rate of experiment 2 was slightly higher than other two experiments (experiment 3 and experiment 4, Table2).
Although direct feeding produced the best results, volunteers are not always easy to find. Thus, researchers are forced to use blood samples that have been stored in refrigerator for an extended period of time. However, this problem will be resolved if Korean isolate which causes vivax-malaria can be cultured successfully.
The present study demonstrated that blood samples that had been stored in refrigerator for 48 hours still produced sporozoites in mosquitoes. However, the positive rate decreased according to the duration of storage in refrigerator. It is possible that the mature gametocytes lost their infectivity after long-term storage under artificial conditions (i.e., in refrigerator). Based on optical density values obtained with an ELISA, the number of sporozoites in infected mosquitoes ranged from 648 to 1,056 (n=8, mean=818). The mean number of sporozoites was lower than the results of Wirtz et al. (1985). In this study, a numbers of sporozoites obtained when a North Korean strain of P. vivax fed to An. gambiae and An. maculatus. Based on the values calculated using a hemocytometer, the average numbers of salivary gland sporozoites were approximately 3,500 in An. gambiae (n=10) and 2,300 in An. maculatus (n=10) (Wirtz et al., 1985). These different results may come from techniques or susceptibility of vectors even though used the same monoclonal antibody. Therefore, we will perform with dissection method in order to figure the abilities of sporozoites production and the number of sporozoites in salivary glands of An. sinensis and compare with the number of sporozoites obtained in this study.
ACKNOWLEDGEMENT
Authors are deeply grateful to Dr. RA Wirtz, CDC, USA, for his offering the monoclonal antibodies against Plasmodium vivax sporozoites.
Notes
This study was supported by a grant of the Korea Health 21 R & D Project, Ministry of Health and Welfare, Republic of Korea (HMP-99-M-04-0002).