Five cases of ocular toxocariasis confirmed by serology
Article information
Abstract
We report 5 cases of ocular toxocariasis in Korean adults complaining of visual impairment along with floating or bubbling sensation. Fundoscopic examination revealed a retinal detachment along with exudate in 4 cases. They all showed typical reaction by ELISA and immunoblot against Toxocra excretory-secretory antigen. One case showed high level of anti-Toxocara IgE antibodies (34,000 Toxocara units/L) as well as increased level of serum total IgE antibodies and the specific IgE antibodies for 3 inhalant antigens, suggesting that high level of anti-Toxocara IgE antibodies was associated with an atopic status. Clinical manifestations were improved after the sequential use of steroids then mebendazole. We also suggest that ocular toxocariasis should be thoroughly investigated even when an evocative uniocular inflammatory lesion is encountered in peripheral retina without a systematic disease.
INTRODUCTION
Human toxocariasis is a zoonotic parasitic disease caused by Toxocara canis or Toxocara cati larvae. Human infection is usually an outcome of accidental ingestion of the embryonated eggs, due to geophagia, pica, and the consumption of contaminated raw vegetables, and poor personal hygiene, especially in childhood. The clinical disorder caused by the migration of Toxocara larva to the eye is currently known as ocular larva migrans. A definite diagnosis of ocular larva migrans is difficult to establish since the larva is rarely identified from the lesions; however, a single case of ocular toxocariasis from a 28-year old woman complaining of a sudden onset of nasal side field defect of the right eye had been reported previously (Park et al., 1999). We hereby report 5 more cases of ocular toxocariasis confirmed by ELISA with specific anti-Toxocara IgE antibodies, together with immunoblot that uses specific IgG antibodies. Moreover, a serial treatment of steroid and mebendazole and the relationship between high titers of anti-Toxocara IgE and the total IgE antibodies also were discussed.
CASE RECORD
Patients
Five cases (4 males and 1 female) of ocular toxocariasis in Korean adults, diagnosed from October 1998 to January 1999 in Kangdong Sacred Heart Hospital, Seoul, Korea, were subjected. A 68-year old male with a loss of his visual acuity in the left eye, together with a complaint of floating sensation was referred to our Department on November 3, 1998. No specific problem was noticed in his past medical history. He had been in close contact with dogs (case 1). A 42-year old male without any particular past medical history attended our clinic due to a floating sensation which had arisen 1-week before, on October 23, 1998. He had a previous history of being exposed to a puppy about 2 to 3 yrs ago (case 2). A 45-year old female, who suffered 2 years from a loss of visual acuity in the left eye together with a floating sensation, attended our consultation on January 6, 1999. Neither a particular past medical history nor the habit of keeping pet animals was recognized (case 3). A 29-year old male presenting a sudden decline of visual acuity in the right eye, which had occurred one year ago, attended our consultation on January 13, 1999. His past medical history was not significant. He had no contact with any pet animals (case 4). A 31-year old male patient presented a decreased visual acuity in the left eye together with a bubbling sensation, which had been developing for 1 year prior to his first visit. The patient attended our consultation on January 26, 1999. No particular past medical history was noticed, except that he had been rearing a puppy in the house for 2 yrs (case 5). All of the five cases did not complain of any hepatobiliary symptoms.
Ophthalmologic examination
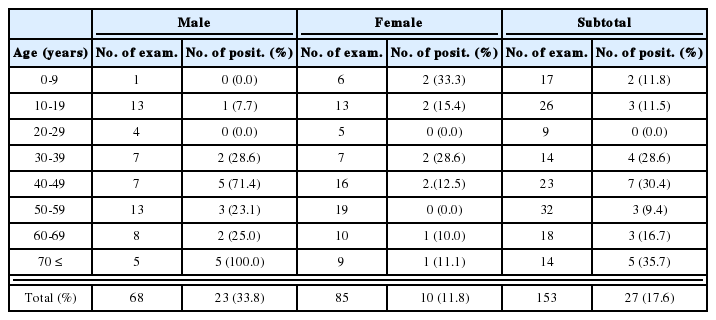
Table 1 summarizes clinical manifestations of the patients. Case 1 showed the visual acuity of 20/20 (left eye) and 20/200 (right eye), which was not correctable. Fundic examination of the left eye showed a tractional retinal detachment with inflammatory exudate, located from 5- to 10-o'clock positions (Fig. 1). In Case 2, the examination of the left fundus found peripapillary inflammatory lesions (Fig. 2). In Case 3, vitreous reactions were found in the left eye. A fundoscopic examination of the left eye showed an inflammatory snowbank-like exudate in inferonasal peripheral retina (Fig. 3). In Case 4, a slit-lamp examination of the right eye showed a normal anterior segment, but a marked infiltration of the vitreous with inflammatory cells. He had a relative afferent pupillary defect in the right eye. With an indirect ophthalmoscopy, a total retinal detachment was noted throughout the entire right retina. A dense, white, and gliotic mass was located in the inferotemporal peripheral retina, between 6- and 7-o'clock positions. In Case 5, the left eye showed marked infiltration of the vitreous and retrolental snowball opacity. The examination of the left fundus revealed tractional retinal detachment with a dense, grayish-white peripheral retinal exudate and fibrovascular membrane between the 3- and 6-o'clock positions (Fig. 4).
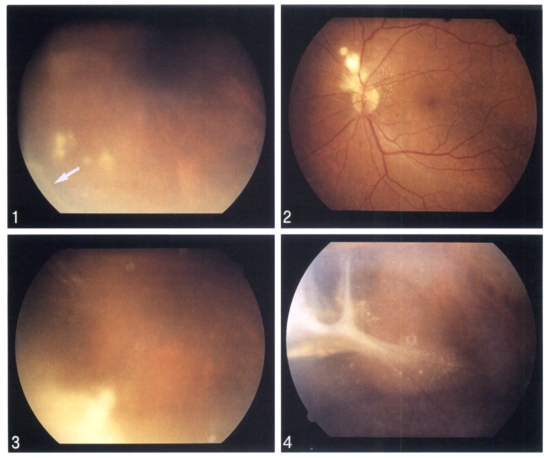
Fundoscopic findings of the cases. Fig. 1. Tractional retional detachment with inflammatory exudate in inferonasal peripheral retinal and relationship to vitreoretinal traction band (arrow) in case 1. Fig. 2. Peripapillary, superonasal to the disc, inflammatory granulomatous lesion in case 2. Fig. 3. A dense inflammatory exudate (snowbank) in inferonasal peripheral retina in case 3. Fig. 4. Tractional retinal detachment with exudate and prominent fibrovascular vitreoretinal band in inferotemporal peripheral retina in case 5.
ELISA, SDS-PAGE and immunoblotting
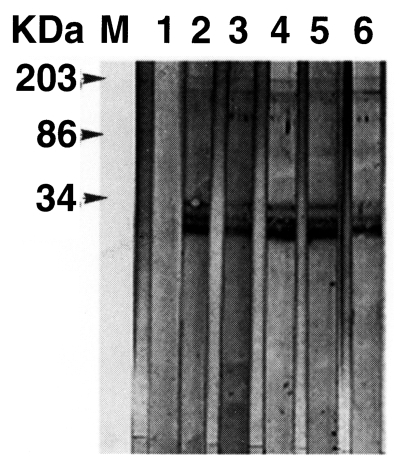
Secretory IgE(sIgE) ELISA was performed using Toxocara excretory antigen (TES-Ag, Toulouse, France) as previously described (Magnaval et al., 1992). For immunoblot analysis, the TES-Ag preparations were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech Inc., Piscataway, NJ) in a semi-blotter (Hoffer, San Francisco, CA). The strips were then incubated with sera diluted at 1:50. An alkaline phosphatase-conjugated anti-human IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted at 1:3,000 was used to detect the immunoreaction. Nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Sigma Co. St. Louis, MO) were used as chromogenic substrates. Positive reactions were verified by 4 low-molecular-weight bands at 24, 28, 30, and 35 kDa, and 3 high-molecular-weight bands of 132, 147 and 200 kDa, respectively (Magnaval et al. 1991). The detection of anti-Toxocara specific IgE antibodies in the patients sera resulted in 34,000, 28, 15, 108, and 10 Toxocara unit (TU), respectively. They were all in the positive range (positive criterion: 5 TU) (Table 2). The patients sera were subjected to immunoblot with TES-Ag. All sera examined showed typical reactions with the 7 bands at 24-28(fused bands), 30, 35, 132, 147, and 200 kDa, respectively (Fig. 2). The test, simultaneously done in France, also yielded the exact same result (data not shown).
Total IgE antibodies and simultaneous multiple allergen tests
The total IgE antibodies levels in the patient sera were measured by IgE radioimmunoassay according to conditions recommended by CAP system (Amersham Pharmacia Biotech Inc., Piscataway, NJ). For the detection of atopic status, MAST-CMA(chemiluscence assay) allergen-specific IgE assay-Korea inhalation panel, composed of 36 allergens common in Korea, was used (MAST Immunosystems, Mountain View, CA). These two tests were done on September 7, 1999, 8 to 11 months after the first visit from cases 1,3, 4 and 5. The serum levels of total IgE antibodies were elevated in Cases 1, 4, and 5 which ranged between 3116-179 IU/mL (Table 1). In allergen-specific IgE assay, Case 1 exhibited a very high serum level against cockroach mix (> 300 Lu), crab (> 300 Lu) and shrimp (> 300 Lu), and showed a moderate level against timothy grass (93 Lu), rye (76 Lu), and a high level against mite-farinae (187 Lu), mite-pterony (194 Lu). Others were in non-detectable range. Case 3 was equal or under non-detectable level against 36 inhalant allergens. The fourth case showed a very high serum level against the cockroach mix (> 300 Lu), and equal or under non-detectable level against others. Case 5 showed a moderate level against mite-farinae (134 Lu), mite-pterony (91 Lu) and equal or under non-detectable level against others.
Other examination
Complete blood count, stool examination for helminth and protozoan parasites and other routine laboratory examinations of biochemical study including liver function test were simultaneously carried out to rule out the possible associated with the disease. All laboratory data were within normal range except for 1 patient who showed eosinophilia of 29.1% (3,340 cells/L, case 1, Table 1). Especially, the result of liver function test from all of five cases was within normal limit.
Treatment of the patient
The patients were given a dose of 1 mg/kg (body-weight) prednisolone daily for 1 month, after which they were prescribed with mebendazole (25 mg/kg body-weight daily) for the following 1 month. Pars plana vitrectomy was done in Case 1 after the treatment, and for Case 5, it was done during the treatment. Improvement of clinical status was found in all 5 cases.
DISCUSSION
In this presentation, we report 5 cases of ocular toxocariasis confirmed by positive antibody reactions to both sIgE ELISA and immunoblot. For the serodiagnosis of ocular toxocariasis, banding patterns of the immunoblot that detect IgG antibodies for TES-Ag are more specific than that of sIgE ELISA. The sIgE ELISA alone is not sufficient for properly ensuring the diagnosis of toxocariasis. The immunoblotting banding patterns that include low-molecular weight bands appear to be specific for toxocariasis; high molecular weight bands are found to be present at significant levels only when sera from patients with various helminthic diseases are tested which TES ELISA show 33% positive results (Magnaval et al., 1991). Although we were not able to find a larva from the cases showing only intra-ocular inflammation, the serological evidences indicated that the larval Toxocara also affected these patients. Immunoblot findings of sera from five patients all revealed low molecular weight bands from 24 to 35 kDa that were specific to toxocariasis.
Furthermore, sIgE ELISA is a complementary method for the detection of specific IgG antibodies. Its sensitivity and specificity are 67. 9% and 76.2%, respectively, when the cut-off value is 5 TU. Specific anti-Toxocara IgE antibodies determination is also useful for the post-treatment follow-up. If elevated prior to therapy, the level significantly decreased.
There may be a possibility of cross reaction with other helminthic infections. However, although the sIgE ELISA for TES-Ag is positive in sera of some other helminthic infections, the immunoblot findings must be either negative, or positive with respect to high molecular bands. Therefore, the possibility of cross reaction with other helminthic infections can be easily excluded (Magnaval et al., 1992).
Ocular toxocariasis usually occurs in 4- to 8-year old otherwise healthy children, and is limited to one eye, and infected by one larva (Schlaegel, 1978). There are a few clinical reports of retinal lesions in adults due to Toxocara infection. Detection of these five cases of adult ocular toxocariasis during 3 months in one hospital is not an usual finding. It is also interesting that the case 1 has an atopic status along with the ocular toxocariasis when judged by high serum levels of Toxocara specific IgE and total IgE antibodies (34,000 TU, and 3,116 IU/mL, respectively) which show very high positive reactions to 3 inhalant antigens. Such case could be classified as a poly-sensitized patient. Although assays for total IgE antibodies and for other allergens were performed after the treatment, influencing the immunological status of the patients, thus possibly decreasing the value, it could be quite possible that the atopy might act as a worsening factor for ocular toxocariasis. The immune response to the local secretion of TES-Ag, one of which is a potent allergen, would be boostered in the atopic patient. Such a hypothesis, regarding respiratory tract troubles, has been previously assessed in a case-control study, leading to a positive reply (Buijs et al., 1997).
We classified the clinical manifestations into four types tentativley. The first and perhaps the best known and the most common destructive form of ocular toxocariasis is a diffuse chronic endophthalmitis. It usually occurs in 2- to 9-year-old and inflammation may not be the prominent feature of ocular toxocariasis. The lesion is usually discovered during an evaluation of strabismus, decreased vision or while undergoing a routine examination. Like the case 4, it typically presents granulomatous keratic precipitates, hypopyon, extremely turbid vitreous with an exudative retinal detachment. It implies a poor visual prognosis and may be an indication for early surgery. In experience, diffuse chronic endophthalmitis is the condition most commonly confused with retinoblastoma. Computed tomography and B-scan ultrasonography usually show a typical tumor pattern in retinoblastoma with evidence of calcium in the mass.
The Second type of clinical manifestations of ocular toxocariasis is a posterior retinochoroiditis or posterior pole granuloma. This form of infestation may initially present relatively hazy vitreous body and sign of acute inflammation, in which the posterior pole granuloma is observed as an ill-defined hazy mass with surrounding vitreous inflammation. Subretinal or intraretinal inflammatory masses in this form of disorder typically become very well-defined and are relatively small, ranging from 0.75 to 6.0 mm in diameter in size. The prognosis of Toxocara endophthalmitis is relatively good.
Third type is a peripheral retinochoroiditis, forming a peripheral inflammatory granulomatous mass. A dense white inflammatory mass is localized. Alternatively, the inflammation may be much more diffuse and appears as a "snowbank" as is visualized in typical severe pars planitis. Fibrocellular bands may be observed running from a peripheral inflammatory mass to the more posterior retina or the optic nerve. Intravitreal traction bands associated with this disorder can lead to production of both traction and rhegmatogenous retinal detachments. The cases 1, 3 and 5 respond to the third type of ocular toxocariasis.
The fourth type of ocular toxocariasis is an optic papillitis, like in the case 2. It is a localized inflammatory reaction in the optic disc. It is characterized by an elevation of the optic disc with telangiectasia of the blood vessels and sometimes subretinal exudation (Ryan, 1994). In addition to these four clinical manifestations, motile nematode larvae may wander aimlessly through the ocular tissues similar to the manner in which it wanders through the viscera in visceral larva migrans. There is a diffuse unilateral subacute neuroretinitis which eventually leads to atrophy of the optic nerve head and causes severe pigmentary derangement throughout the fundus (Suja and Nakshima, 1995).
There was no hepatobiliary symptom reported from five patients. Also the liver function test was within normal limit. It was due to the fact that the number of larvae was not enough to induce the lesion in the liver, although the larvae was introduced to liver in adults.
Mebendazole treatment had a down-regulatory effect on the serum levels of specific anti-Toxocara IgE antibodies, while those of total IgE antibodies were not affected either with the use of diethylcarbamazine or by mebendazole (Magnaval, 1995). In this respect, corticosteroid therapy helps to reduce the inflammatory process without permitting the overgrowth of the infectious agent. Pars plana vitrectomy and retinal detachment surgery have been used in the case of ocular toxocariasis to remove the vitreous opacity caused by chronic inflammation and to relieve the vitreoretinal traction and retinal detachment. Out of 5 cases, pars plana vitrectomy was done in cases 1 and 4 (Werner et al., 1999).
The fact that only 5 cases of ocular toxocariasis were diagnosed within a short duration suggest the possibility of more ocular toxocariasis cases in Korea. We need to pay more attention to these larva migrans. This is the first serial of sequential use of steroid and then mebendazole with good results. We suggest that ocular toxocariasis should be further investigated even when there are no specific findings of a systemic evaluation with specific uniocular inflammatory lesion in the peripheral retina.
ACKNOWLEDGEMENTS
We appreciate Mr. Yong Taek Chang, President of Shinpoong Pharmaceutical Co. Ltd., Seoul, Korea, for his generosity and kindness in providing us with mebendazole (Ebendazole®) for treatments.
Notes
This paper was presented as a poster at the 82nd Annual Meeting of the Korean Ophthalmological Society in Yongpyong, Korea, on April 16, 1999.