The 10 kDa protein of Taenia solium metacestodes shows genus specific antigenicity
Article information
Abstract
Genus specific antigenicity of the 10 kDa protein in cyst fluid (CF) of Taenia solium metacestodes was demonstrated by comparative immunoblot analysis. When CFs from taeniid metacestodes of T. saginata, T. solium, T. taeniaeformis and T. crassiceps were probed with specific monoclonal antibody (mAb) raised against 150 kDa protein of T. solium metacestodes, specific antibody reactions were observed in 7 and 10 kDa proteins of T. solium and in 7/8 kDa of T. saginata, T. taeniaeformis and T. crassiceps. The mAb did not react with any protein in hydatid fluid of Echinococcus granulosus and E. multilocularis. This result revealed that the 10 kDa peptide of T. solium metacestodes and its equivalent proteins of different Taenia metacestodes are genus specific antigens that are shared among different Taenia species.
Neurocysticercosis (NCC), an infection of the central nervous system caused by Taenia solium metacestodes, is recognized as a major cause of neurological disease worldwide. The disease is now regarded as one of the most important emerging diseases together with other viral and drug resistant bacterial infections (White, 1997).
Neuroimaging findings from several neurological patients provide a clue leading to the diagnosis of NCC. In many patients diagnosed with NCC, however, supplementary immunological tests were necessary to support the diagnosis because neuroimaging findings often failed to distinguish NCC from other brain diseases (Chang et al., 1988). A specific antigen is important for undertaking the serological tests. Cyst fluid (CF) of T. solium metacestodes is currently recognized as the most reliable sources of diagnostic antigens (Cho et al., 1988; Yang et al., 1998). Recently, a 10 kDa peptide of 150 kDa protein in the CF appeared to be sensitive and specific in diagnosing active NCC (Yang et al., 1998; Chung et al., 1999).
Many investigators have been searching for a substitute antigen in serodiagnosis of NCC since T. solium metacestodes are difficult to collect even in highly endemic areas. In addition, CFs of T. crassiceps, T. saginata and T. hydatigena have been regarded as substitute antigens, because they were found to share common immunological responses against NCC (Hayunga et al., 1991; Zarlenga et al., 1994; Bueno et al., 2000). However, to understand the common antigenicity shown among different speices, further studies are required with respect to the common epitopes which are shared not only by CF of T. solium and other Taenia species but also by hydatid fluid (HF) of Echinococcus granulosus and E. multilocularis. In the present study, we have examined whether the 10 kDa glycoprotein of T. solium metacestodes shares common antigenicity in CF/HF of several different taeniid metacestodes.
CF of T. solium metacestodes was collected from naturally infected pigs as described previously (Yang et al. 1998). Metacestodes of T. saginata were collected from an experimental calf which was challenged with 100,000 viable eggs. Metacestodes of T. taeniaeformis and T. crassiceps were harvested from laboratory maintained rats and mice, respectively. HF of E. granulosus was obtained from a patient with cystic echinococcosis. HF of E. multilocularis was collected from an experimental rat. The crude CFs and HFs were centrifuged at 20,000 g for 1 hr and the supernatant was used as antigens. All procedures were carried out at 4℃. They were stored at -70℃ until use further.
Monoclonal antibody (mAb) was generated by immunizing BALB/c mice using the 150 kDa protein from native CF of T. solium metacestodes (Cho et al., 1988). Antibody secreting cells specific to 7, 10 and 15 kDa of 150 kDa proteins were selected and expanded. IgG fractions were purified by Protein-A Sepharose 4B affinity column (Phamarcia, Piscataway, NJ, USA). CFs and HFs from different species were separated by 10% Tricine SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The blot was incubated with mAb diluted at 1:200. Peroxidase conjugated anti-mouse IgG (whole molecule, Cappel, PA, USA) was used in dilution of 1:1,000. The blot was developed with 4-chloro-l-naphthol chromogen (Sigma, St. Louis, MO, USA).
Fig. 1A shows a protein profile resolved by Tricine SDS-PAGE. CF of T. saginata metacestodes exhibited four major bands between 40-60 kDa, but no prominent proteins in a low molecular weight range were observed. Only the 7 and 10 kDa proteins could be faintly identified (lane a). CF of T. solium metacestodes demonstrated several protein bands including 7, 10, and 15 kDa as major constituents (lane b). Those of T. crassiceps and T. taeniaeformis showed similar profiles in which 7 or 8 kDa protein was the major component (lanes d and e). HF of E. granulosus and E. multilocularis exhibited major bands at 52 and 43 kDa. In addition, a 8 kDa protein, which may be a subunit of E. granulosus antigen B (Fernandez et al., 1996), was observed in E. granulosus (lane c). The same blot was probed with generated mAb. As shown in Fig. 1B, CF of T. solium metacestodes reacted strongly to 7 and 10 kDa proteins (lane b). CF from T. saginata reacted strongly to a 7 kDa protein while reacting weakly to a 10 kDa protein (lane a). CFs of T. taeniaeformis (lane d) and T. crassiceps (lane e) showed reactive band each at 7 or 8 kDa with minor difference in their molecular weights. In contrast, the mAb did not recognize any reactive band in HFs of E. granulosus or of E. multilocularis (lanes c and f).
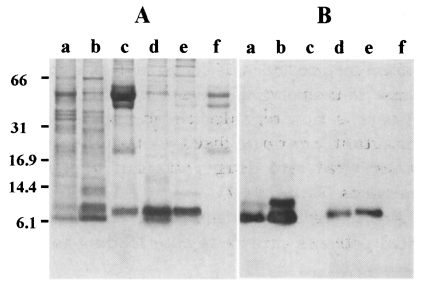
(A) Analysis of the CF proteins resolved by 10% Tricine SDS-PAGE stained with Coomassie blue. (B) Immunoblot analysis of CF/HF from metacestodes of different taeniid cestodes with anti-150 kDa mAb of T. solium metacestodes. The CFs are probed with 1:200 diluted antibody. CFs from metacestodes of T. saginata (lane a), T. solium (lane b), T. taeniaeformis (lane d) and T. crassiceps (lane e) show immunoreactive bands at around 7-10 kDa. On the contrary, those from E. granulosus (lane c) and E. multilocularis (lane f) do not show positive reactions.
The present observation demonstrated clearly that the 7 or 8-10 kDa proteins are common components present mostly in CFs of different Taenia species metacestodes. As shown in Fig. 1A, there were two distinctive bands corresponding to 10 and 7 kDa in molecular weights in CF of T. solium metacestodes while only one band, approximately 7 or 8 kDa in size, was found in other Taenia species. The proteins had previously been described as 10 kDa possibly due to a different degree of glycosylation as well as to a low resolving power of conventional SDS-PAGE that failed to accurately determine the molecular weights (Hayunga et al., 1991; Zarlenga et al., 1994; Chung et al., 1999). In fact, the proteins were assumed to be 7 or 8 kDa judged by Tricine SDS-PAGE as shown in this study.
The mechanism of common antigenicity shared between the 10 and 7 kDa proteins in CF of T. solium metacestodes is poorly understood. A possible explanation is that the two proteins may be a subunit of the 150 kDa protein complex which shares a high degree of sequence homology. Elucidation of molecular information of these related genes will likely to resolve this hypothesis.
The 10 kDa antigen identified in previous and present studies (Yang et al., 1998; Chung et al., 1999) seems to be same with the 8 kDa peptide described by Rodriguez-Canul et al. (1998). It is also possible that the 10 kDa protein is a reduced form of glycoprotein of 24 and 39-42 kDa (Tsang et al. 1989) as analyzed by Plancarte et al. (1999). Clearly, the 10 kDa antigen of T. solium metacestodes or 7 or 8 kDa proteins with similar diagnostic properties present in other Taenia species could be valuable in strengthening clinical diagnosis of NCC. With this 10 kDa protein, the differential diagnosis of cysticercosis from both cystic and alveolar echinococcosis may be possible.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. S. Geerts, Institute of Tropical Medicine, Belgium who kindly sent us T. saginata viable eggs. Dr. A. Ito, Asahikawa Medical College, Japan is also acknowledged for his generous donation of HF of E. multilocularis metacestodes.
Notes
This work was supported by a research grant from Sungkyunkwan University School of Medicine (1998-1999).