Partial characterization of a 17 kDa protein of Clonorchis sinensis
Article information
Abstract
A 17 kDa protein from Clonorchis sinensis adults was purified by a procedure including Sephacryl S-200 HR gel filtration and Q-Sepharose anion exchange chromatography. The protein was proved to be a cysteine protease as it showed hydrolytic activity toward Cbz-Phe-Arg-AMC in the presence of dithiothreitol and was inhibited by specific inhibitors such as iodoacetic acid or trans epoxy-succinly-L-leucyl-amido(4-guanidino) butane. The polyclonal antibody raised against the protein reacted to 17 kDa proteins of trematodes such as Paragonimus westermani, Fasciola hepatica, Opisthorchis viverrini, Gymnophalloides seoi, and Metagonimus yokogawai. The antibody recognized the 17 kDa and 16 kDa cysteine proteases purified from C. sinensis, P. westermani, and G. seoi as well. These results suggest that the 17 kDa protein may be a cysteine protease commonly present in trematodes.
Parasite proteases are known to play pivotal roles in tissue penetration, nutritional uptake, and immune evasion from the host (McKerrow, 1989). Amongst these, the cysteine proteases show diverse roles in migration, nutrients uptake, and immune evasion (Kong et al., 1995; Chung et al., 1997; Choi et al. 1999). The 17 kDa protein of Clonorchis sinensis had previously been reported as a cysteine protease (Song et al., 1990), although its precise characteristics have not been elucidated. Previous SDS-polyacrylamide gel electrophoresis studies showed that various species of helminths possess 17 kDa protein bands and a 17 kDa protein of Paragonimus westermani was reported as a cysteine protease (Chung et al., 1997), and 16 kDa cysteine proteases were found from Nippostrongylus brasiliensis and Gymnophalloides seoi (Kamata et al., 1995; Choi et al., 1998). In the present study, we partially characterized a 17 kDa protein of C. sinenisis and demonstrated the 17 kDa protein could be a common protein present in various trematode parasites.
The procedures for worm collection and preparation of C. sinensis crude extracts were carried out as described elsewhere (Hong et al., 1999). Purification of the 17 kDa protein was performed by a two-step column chromatography employing the methods of Chung et al. (1997) including Sephacryl S-200 HR gel filtration and Q-Sepharose anion exchange chromatography. Proteolytic assay was measured fluorometrically using synthetic dipeptide substrate carbobenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (Cbz-Phe-Arg-AMC; Sigma) with 2 mM dithiothreitol (DTT). One unit of the enzyme activity was determined as the amount of the enzyme that caused releasing of 1 µM of AMC in 1 hr (Chung et al., 1997). The enzyme inhibition test was measured with specific inhibitors such as iodoacetic acid (IAA, 20 µM), trans epoxy-succinly-L-leucyl-amido(4-guanidino) butane (E-64, 10 µM), diisopropylfluorophosphate (DFP, 2 mM), 3,4-dichloroisocoumarin (3,4-DCI, 10 µM), 4-(amidinophenyl) methanesulphonyl fluoride (APMSF, 10 µM), ethylenediamine tetraacetic acid (EDTA, 2 mM). All chemicals were purchased from the Sigma. The immune sera of BALB/c mice raised against the 17 kDa protein were prepared by the methods described by Kong et al. (1997). The purity of the enzyme was analyzed by 7.5-15% SDS-PAGE under reducing condition. Immunoblotting was performed using an anti-17 kDa antibody and antigens of other parasites. After electropho-resis, the protein bands were transferred onto polyvinylidene difluoride membrane (PVDF, Amersham Pharmacia, Uppsala, Sweden). The membrane was incubated overnight with 1:1,000 diluted anti-17 kDa antibody at a room temperature and followed by incubation with 1:1,000 diluted peroxidase-conjugated anti-mouse IgG for 3 hr. The blots were developed with 4-chloro-1-naphtol.
SDS-PAGE revealed that the purified protein from C. sinensis migrated as a 17 kDa (Fig. 1). The protein cleaved Cbz-Phe-Arg-AMC in the presence of DTT and was completely inhibited by cysteine protease specific inhibitors, IAA and E-64, but not inhibited by serine or metalloprotease inhibitors such as DFP, APMSF and EDTA (Table 1). These results indicated that the purified enzyme belongs to the cysteine protease family.

Purification of a 17 kDa protein bands of Clonorchis sinensis analyzed by 7.5-15% SDS-PAGE. 1, Crude extracts; 2, purified 17 kDa protein. Molecular weight in kDa is indicated.
Immunoblot analysis of the crude extracts from other parasites revealed that the anti-17 kDa antibody reacted with the 17 kDa bands of many other trematodes such as C. sinensis, Opisthorchis viverrini, P. westermani, Fasciola hepatica, G. seoi and Metagonimus yokogawai (Fig. 2). In addition, the antibody was specifically recognized purified 17 kDa cysteine proteases from C. sinensis and P. westermani, the 16 kDa cysteine protease of G. seoi, but not the 16 kDa cysteine protease of N. brasiliensis (Fig. 2). The antibody, however, failed to detect any proteins from C. sinensis metacercarial extracts and Schistosoma japonicum extracts (data not shown). These results suggest that the 17 kDa protein is commonly shared by many trematodes but possibly not with cestodes or nematodes. The 17 kDa protein may be a cysteine protease that commonly present in different trematode parasites.
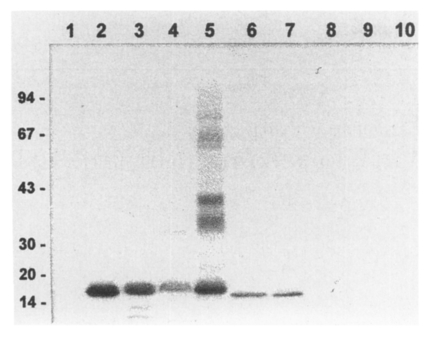
Common presence of a 17 kDa protein in different trematode parasites analyzed by immunoblotting. 1, metacerarial extracts of C. sinensis; 2, crude extracts of C. sinensis; 3, crude extracts of O. viverrini; 4, crude extracts of F. hepatica; 5, crude extracts of P. westermani; 6, crude extracts of G. seoi; 7, crude extracts of M. yokogawai; 8, crude extracts of S. japonicum; 9, sparganum crude extracts; 10, crude extracts of N. brasiliensis.
Notes
This study was supported in part by the grant number 04-98-051 Hospital Research Fund from the Seoul National University.
