Non-specific activation of mouse peritoneal macrophages by a freshwater ciliate, Tetrahymena pyriformis
Article information
Abstract
Toxoplasma-killing activities of mouse peritoneal macrophages activated by the extracts of Tetrahymena pyriformis (Korean and Chinese strains) were evaluated, and the active protein fractions from both strains were partially characterized by a method including chromatographies and SDS-PAGE. The first peak in Korean strain and the second peak in Chinese strain of T. pyriformis obtained by DEAE-Sephadex A-50 chromatography were most effective in the activation of macrophages to kill Toxoplasma gondii tachyzoites in vitro. Subsequent fractionations of obtained peak fractions were performed on a Sephadex G-200 gel. The first peaks fractionated from both strains of T. pyriformis had the highest toxoplasmacidal activities, and when subjected to the SDS-PAGE, one prominent band was visualized for each of the strains showing the same molecular weight of ca. 52.6 kDa. This active protein is suggested to be related to non-specific activation of mouse peritoneal macrophages.
INTRODUCTION
A freshwater ciliate Tetrahymena pyriformis has been widely used as experimental materials in the fields of cell biology and biochemistry, because it was known that the organism proliferates well in large quantities in an axenic culture condition. In immunological aspect, it has been known that Tetrahymena treatment into mice could produce an increased non-specific resistance against a subsequent Toxoplasma gondii challenge infection (Makioka et al., 1982; Makioka and Kobayashi, 1983, 1984, 1985).
Mycobacterium bovis BCG (Senterfitt and Shands, 1970), Corynebacterium parvum (Swartzberg et al., 1975; Krahenbuhl et al., 1976) and Freund's complete adjuvant (Remington et al., 1972) have been already recognized as non-specific immunostimulators. The candidacidal activity of murine peritoneal cells in vitro was studied to observe the non-specific microbicidal activity of phagocytes after intraperitoneal injection of mice with different non-biological synthetic activators such as methylamine, heparin, polyol L, suramin, dextran sulfate, dimethyldioctadecyl-ammonium bromide (DDA) and liquoid, etc. (Hilgers et al., 1985).
An in vitro culture system of T. gondii in dimethylsulfoxide (DMSO)-induced HL-60 cell line was made by Choi et al. (1988), and they found that the increased phagosome-lysosome fusions in the host cells eventually cause the killing of the target parasites. In addition to this functional killing mechanism, interferon-γ (IFN-γ) was objected as a modulator of toxoplasmacidal activity in the macrophages (Lee and Shin, 1994; Soh et al., 1996). Recently, bacterial lipopolysaccharide (LPS) was reported as a potent activator of the macrophage (Barbour et al., 1998), and the tumor growth factor-β (TGF-β) response was characterized in alveolar macrophage activation during pulmonary inflammation and fibrosis (Matrat et al., 1998).
A single active protein fraction was detected from T. pyriformis (W strain) which could effectively activate macrophages having the ability to kill T. gondii in vitro. The molecular weight of the single protein fraction was 64 kDa. However, the other proteins such as bovine serum albumin, pepsin, and muramyl dipeptide (MDP) were not able to induce the activation of macrophages (Makioka and Kobayashi, 1986). Peritoneal macrophages from mice administered with the Korean strain of Tetrahymena exhibited significant resistance against T. gondii infection as compared to those treated with synthetic activators (Kim et al., 1991). Among non-biological synthetic activators, DDA was evaluated as an excellent activator. However, they have not demonstrated to observe the strain-specificity of T. pyriformis and to carry out the biochemical characterization of active protein fractions that have a significant protective ability.
The present study was carried out to determine whether or not the intraperitoneal administration of Korean and Chinese strains of T. pyriformis into mice activate murine peritoneal macrophages to kill T. gondii tachyzoites in vitro, to obtain the active protein fraction(s) from T. pyriformis having a significant toxoplasmacidal activity in the host cells, and to observe any specific differences of active fractions between two strains of Tetrahymena.
MATERIALS AND METHODS
Parasite employed
Virulent tachyzoites of RH strain of T. gondii were serially passaged in inbred ICR mice (15-20 g in body weight) every 3-4 days. The peritoneal exudates were harvested from the mice and suspended in phosphate-buffered saline (PBS, pH 7.4). The supernatant of T. gondii suspension was obtained by the first centrifugation at 30 g for 5 min. Tachyzoites were purely isolated by the second centrifugation at 270 g for 10 min, and then rinsed twice with PBS.
Activators of murine peritoneal macrophages
The Korean strain of T. pyriformis (GL strain) was obtained from the Department of Parasitology, Inha University College of Medicine, and the Chinese strain of T. pyriformis was from the University of Beijing, Beijing, China. Both strains were cultivated in PYD medium containing 1% proteose peptone, 0.5% yeast extract, and 0.87% dextrose (Watanabe and Ikeda, 1965; Makioka and Kobayashi, 1985). Tetrahymena pyriformis were serially subcultured in medium to medium every one month.
The organisms were harvested by centrifugation at 850 g for 5 min and washed twice with an inorganic medium (NaCl, 100 mg; KCl, 4 mg; CaCl2, 6 mg in one liter of distilled water). A total of 5 × 107 Tetrahymena trophozoites was sonicated at grade 60 with PBS (pH 7.4), and the supernatant to be used as activator was obtained by centrifugation at 28,000 g for 30 min. Protein assay was made by the method of Lowry et al. (1951).
To obtain the water-soluble protein fractions, the following procedures were performed as described by the method of Makioka and Kobayashi (1986). The harvested Tetrahymena trophozoites (5 × 107) were washed twice by centrifugation at 800 g with an inorganic medium. Packed Tetrahymena were suspended in nine volumes of distilled water containing 0.1 mM N-α-tosyl-L-lysyl-chloromethane hydrochloride (TLCK, a potent protease inhibitor) and homogenized by sonication. The homogenate was kept standing at 4℃ for more than 3 hr and centrifuged at 28,000 g for 30 min. The centrifuged supernatants were dialyzed against to distilled water, and lyophilized.
The extract of water-soluble protein of Tetrahymena was applied to DEAE-Sephadex A-50 column (2.5 × 32 cm; Pharmacia, Uppsala, Sweden) equilibrated at 20℃ with 20 mM Tris-HCl (pH 7.5). The water-soluble protein extracts (70-80 mg) of T. pyriformis were applied to the column and eluted with a linear gradient of 0-0.3 M KCl. Fractions (4 ml) were collected at a flow rate of 20 ml/hr. The fractions were pooled, dialyzed against to distilled water, and lyophilized. About 10 mg of sample of the most active peak from DEAE-Sephadex A-50 chromatography was applied to a Sephadex G-200 column (2.5 × 86 cm; Phamacia) equilibrated with 20 mM Tris-HCl (pH 7.5) and eluted at a flow rate of 16 ml/hr. Fractions (4 ml) were collected, pooled, dialyzed, and lyophilized.
Proteins in each chromatographic step were analysed on sodium dodecyl sulfate (SDS)-polyacrylamide gels (Laemmli, 1970).
Inoculation
Tetrahymena lysates (590 µg/ml) were inoculated intraperitoneally into 4-5 ICR mice (15-20 g in body weight) five days before harvesting peritoneal macrophages. Control group was treated with 1 ml of saline.
On the other hand, each peak of Tetrahymena extract (590 µg/ml) obtained by ion exchange chromatography was injected intraperitoneally into two experimental mice to observe the activation of macrophages. The fractions showing the most effectiveness were fractionated again by gel filtration, and then each peak (70 µg/ml) was examined for the activation of mouse peritoneal macrophages.
Cultivation of peritoneal macrophages
After 5-day activation of macrophages, 10 ml of Hank's balanced salt solution (HBSS)-10% heat-inactivated fetal calf serum (FCS; Gibco, Grand Island, New York, USA) was injected intraperitoneally into each experimental mouse by a 18-gauze needle, and the abdominal skin was massaged for 2 min, then the peritoneal exudates were obtained by the same syringes, and centrifugated at 270 g for 5 min. The pellets were washed with HBSS-10% FCS twice and pooled. Briefly, 5 × 106 cells were dispensed into 36 × 15 mm plastic petri dish (Nunc, Roskilde, Denmark) and incubated with 5 ml of fresh RPMI-1640-10% FCS medium containing 0.5 µl of antibiotic-antimycotic streptomycin/ml (Sigma) at 37℃ in a 5% CO2 incubator for 2 hr. After removal of nonadherent cells, resident macrophages were obtained. They were incubated in the fresh medium for two days until they formed monolayers
Assay for toxoplasmacidal activity of macrophages
The 5 × 106 tachyzoites of Toxoplasma RH strain were challenged into each experimental group of adherent macrophages. The penetration of Toxoplasma tachyzoites into the macrophages was observed by Giemsa staining after 1 hr incubation. The culture systems were rinsed with RPMI-1640-10% FCS thoroughly after 1 hr incubation to remove extracellular tachyzoites, and then reincubated with fresh RPMI-1640-10% FCS for 20 hr. After reincubation, the petri dishes were washed with PBS (pH 7.4) and stained with Giemsa solution after fixation in absolute methanol. The percentages of the adherent cells infected with Toxoplasma tachyzoites at 1 hr and 20 hr incubation, and total numbers of organisms per 100 macrophages were determined by the following formula (Makioka and Kobayashi, 1986; Kim et al., 1991):

Statistical analysis
Results were expressed as the arithmetic mean±standard error from four experiments with one sample. Student's t-test was applied to analyze statistical significance of the results. The significant order of activators among experimental groups was made by Duncan's analysis as the multiple range test.
RESULTS
Anti-Toxoplasma activities of peritoneal macrophages from mice inoculated with lysates of Tetrahymena
Toxoplasmastatic or toxoplasmacidal actions of murine peritoneal macrophages treated with activators from Tetrahymena lysates were evaluated by percentage of the macrophages infected with live T. gondii tachyzoites (PI), and by total number of tachyzoites in 100 macrophages counted (Tp) in each experimental group (Table 1).
At 1 hr incubation, the PI and Tp values of T. pyriformis (Korean strain) lysate group was shown to 74%, 313 and those of T. pyriformis (Chinese strain) lysate group was shown to 62%, 212. After 20 hr incubation, marked differences of PI and Tp values between the experimental groups were observed. The highest PI and Tp values were detected in control (45%, 215). Sharp reduction of PI and Tp values especially in T. pyriformis (Chinese strain) lysate group was noticeable (4%, 5). These two Tetrahymena experimental groups showed the highly effective toxoplasmacidal activities
Anti-Toxoplasma activities of peritoneal macrophages from mice inoculated with the protein fractions of Tetrahymena from DEAE-Sephadex A-50 chromatography
Fractionation of the water-soluble protein extracts of T. pyriformis (Korean strain) was carried out by column chromatography on Sephadex A-50. Two flowthrough peaks (fractions, 9-22, 28-53) and three binding peaks (fractions, 65-70, 71-78, 79-92) were collected as shown in Fig. 1A.
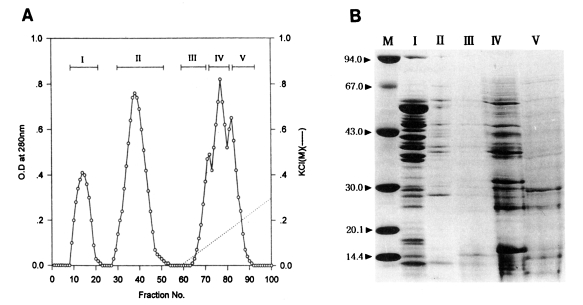
(A) Protein fractions of Tetrahymena pyriformis (Korean strain) on DEAE-Sephadex A-50 chromatography. (B) SDS-PAGE of fractions. M, marker proteins.
Toxoplasmacidal effects of each of the five peaks are shown in Table 2. After 1 hr incubation, the PI and Tp values of experimental groups ranged from 52% to 63%, and 264 to 287, respectively. The control group showed the lowest values (48%, 180). Of the fractions, peak I showed the highest value (63%, 287). After 20 hr incubation, peak I also revealed the sharpest reduction of the values (11%, 19) among fractions, at the first rank of Duncan's order. The values of the other peaks II-V ranged from 46% to 51% (PI) and 327-547 (Tp).
Electrophoretic patterns and their molecular weights determined by SDS-PAGE are shown in Fig. 1B. Numerous protein components, both major and minor, in fractionated peaks were broadly distributed between the molecular weights of 14-67 kDa. Especially, in peak I which showed the highest toxoplasmacidal effect, a total of 17 bands (10 bands of 35-65 kDa, and 7 bands less than 30 kDa) was observed.
Fractionation of the water-soluble protein extracts of T. pyriformis (Chinese strain) was also carried out by column chromatography on Sephadex A-50. Two flowthrough peaks (fractions, 9-17, 27-41) and three binding peaks (fractions, 55-60, 60-68, 69-84) were collected as shown in Fig. 2A.

(A) Protein fractions of Tetrahymena pyriformis (Chinese strain) on DEAE-Sephadex A-50 chromatography. (B) SDS-PAGE of fractions. M, marker proteins.
In the same manners as in Korean strain, toxoplasmacidal effects of each of five peaks inoculated for macrophage activation are shown in Table 3. After 1 hr incubation of activated macrophages with live Toxoplasma tachyzoites, the PI and Tp values of experimental groups ranged 49%-73% and 175-298, respectively. Control group showed the lowest values (48%, 180). The highest values (73%, 245) were obtained in peak II differently from the peaks of Korean strain, in which peak I showed the highest values. After 20 hr incubation, the PI and Tp values of peak II (32%, 55) were remarkably lower than those of control group (41%, 241) as well as the other experimental groups (45%-59%, 131-157). In this respect, the peak II of Chinese strain was regarded as the best peak in terms of Toxoplasma-killing capacity.
Electrophoretic patterns and molecular weights of those peaks on SDS-PAGE are shown in Fig. 2B. Many major and minor protein components in the five peaks were broadly distributed in the range of molecular weights, 14-67 kDa. The peak II, which showed the highest toxoplasmacidal effect, contained two major bands, 40 and 64 kDa.
Anti-Toxoplasma activities of peritoneal macrophages from mice inoculated with the protein fractions of Tetrahymena from Sephadex G-200 gel filtration
In an attempt to further purify an active protein in peak I from the Korean strain and peak II from the Chinese strain of T. pyriformis which showed the highest toxoplasmacidal effects, gel filtration on Sephadex G-200 was carried out. The elution patterns are shown in Figs. 3A and 4A. Three peaks in each strain were separated; fractions, 21-33, 37-52, 54-80 in Korean strain, and fractions, 20-28, 29-56, 57-83 in Chinese strain.

(A) Gel filtration fractions of the peak I from DEAE-Sephadex A-50 chromatography of the protein of Tetrahymena pyriformis (Korean strain). (B) SDS-PAGE of fractions. M, marker proteins.
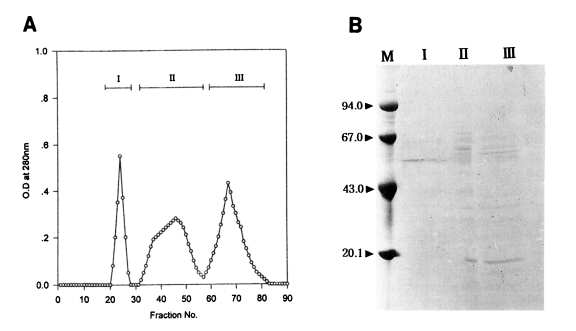
(A) Gel filtration fractions of the peak II from DEAE-Sephadex A-50 chromatography of the protein of Tetrahymena pyriformis (Chinese strain). (B) SDS-PAGE of fractions. M, marker proteins.
Toxoplasmacidal effects of each of these three peaks inoculated for macrophage activation are shown in Tables 4 and 5. After 1 hr incubation of macrophages activated with Korean strain of T. pyriformis and challenged with live Toxoplasma tachyzoites, the peak I showed the highest PI and Tp values (53%, 188) among those of the other peaks, while the values of control group were the lowest (39%, 59). After 20 hr incubation, macrophages activated with peak I exhibited sharp reductions of the PI and Tp values (18%, 43) at the first rank of Duncan's order, even when compared to those of the control (43%, 168).
In the Chinese strain, macrophages activated with peak I showed the highest phagocytic and toxoplasmacidal effects at the first rank of Duncan's order as in the Korean strain. The PI and Tp values of peak I after 1 hr incubation were the highest among the experimental and control groups (peak I, 63% and 162; control, 39% and 59), and the values of peak I after 20 hr incubation were also sharply reduced as compared with control group (peak I, 10% and 36; control, 43% and 168).
Electrophoretic patterns and their molecular weights of the peaks on SDS-PAGE are shown in Figs. 3B and 4B.
In the Korean strain, the peak I from Sephadex G-200 gel filtration showed the best toxoplasmacidal effect, containing a single band (Lane I in Fig. 3B) as revealed by SDS-PAGE. Its apparent molecular weight was 52.6 kDa. Three or four major protein components in the peak I were shown in the range of molecular weights, 35-65 kDa and about ten major protein components in the peak II were broadly distributed in the range of molecular weights, 14-65 kDa. On the other hand, in the Chinese strain, peak I (Lane I in Fig. 4B) showing the highest anti-Toxoplasma activity also revealed the same pattern as the Korean strain containing a single prominent band with molecular weight of 52.6 kDa. Many major and minor protein components in the peaks II and III were shown in the range of molecular weights of 20-67 kDa.
The results of gel filtration of the active fractions indicated that the effective fraction could be separated as the first peak in both strains of T. pyriformis, and each contained a single protein band of 52.6 kDa.
DISCUSSION
The murine peritoneal macrophages activated with Korean strain of T. pyriformis exhibited the excellent Toxoplasma-killing effect much more than activated with synthetic chemical activators (Kim et al., 1991). Therefore, toxoplasmacidal effects of only two Tetrahymena strains (Korean and Chinese) were compared in this study. Also, the water-soluble extracts of Tetrahymena were considered to be more favorable for the evaluation of toxoplasmacidal effects than the KCl-soluble extracts (Makioka and Kobayashi, 1986). Therefore, only the water-soluble extracts of T. pyriformis were fractionated by chromatographies in this study due to the ease in the application for a practical use.
Toxoplasmacidal effect of Chinese strain of T. pyriformis was evaluated with the same mouse model as the former experiments, and the similar results of ability of macrophage activation were observed without any noticeable strain specificity as compared with the Korean strain of Tetrahymena.
Active fractions showing the most toxoplasmacidal effect on DEAE-Sephadex A-50 chromatography were peak I for the Korean strain and peak II for the Chinese strain of Tetrahymena; however, a dark single band with molecular weight of 52.6 kDa was detected on Sephadex G-200 gel filtration in both strains. Unfortunately, electrophoretic result obtained from the Chinese strain was little vague (Fig. 4B) because of technical difficulties. It was confirmed in this study that one of the most strongly macrophage-activating proteins is the 52.6 kDa protein without any strain specificity between the Korean and Chinese strains. Moreover, Japanese strain (W strain) also exhibited the active band with the molecular weight of 64 kDa (Makioka and Kobayashi, 1986).
Hilgers et al. (1985) reported that C. parvum strain 4982 was more effective than strain 10387 in the ability to stimulate the mononuclear phagocytic system for candidacidal activity. As observed by the above authors, minimum dosages of activators, durations of activator effectiveness, and effects of repeated treatment of a single activator or those of combined treatment of more than two activators etc. were not evaluated in this study, although no remarkable parasite-killing effect of Tetrahymena combined with BCG was observed by Makioka et al. (1982).
The capacity of polyanionic agents to activate macrophages was found by Schultz et al. (1977) and these polyanions were suggested to be the consequence of the induction of interferon. However, Hilgers et al. (1985) could not detect an increase of interferon in the effect of candidacidal activity of mouse peritoneal cells. Recently, IFN-γ was the only one of cytokines capable of significantly activating the peritoneal macrophages from Toxoplasma-infected mice (Lee and Shin, 1994). The water extract of Coix lacryma seed activated macrophages in vitro by enhancing nitric oxide (NO) production, and inhibited propagation of the phagocytosed T. gondii (Soh et al., 1994). The highly effective macrophage activators such as Tetrahymena lysates and active protein fractions were also evaluated in this study, but we could not elucidate the Toxoplasma-killing mechanisms in detail. The roles of cytokines from the effector cells and NO production from the activated macrophages have to be studied in the point of establishing toxoplasmacidal mechanisms of Tetrahymena-activated host cells.
The biochemical characterization and clinical application of a single protein (M.W. 52.6 kDa) extracted from T. pyriformis and exhibited an excellent toxoplasmacidal effect in this study, should be investigated in the further studies.
ACKNOWLEDGEMENT
Authors are deeply grateful to Mr. Myung-Kee Hwang and Mr. June-Woo Park for their technical assistances.
Notes
This study was supported, in part, by the 1997 Research Fund of Korea Research Foundation (1997-001-F00066), and the 1995 Research Fund of Inha University.