Is Pneumocystis carinii vertically transmitted to neonatal rats?
Article information
Abstract
Pneumocystis carinii is a pulmonary pathogen of immunocompromised humans or other mammals. Its infection results from activation of organisms involved in latent infection or from new infection through the air. Almost all children are known to be infected within 2 to 4 years of birth, though prenatal transplacental transmission has not yet been demonstrated. In this study we observed experimental P. carinii infection in neonatal rats, thus investigating the possibility of transplacental vertical transmission by Diff-Quik staining of the lung impression smears and in-situ hybridization for lung sections. The positive rate of P. carinii infection in immunosuppressed maternal rats was 100%, but that in normal maternal rats was 0%. Cystic forms of P. carinii were observed in three of six 1-week old neonatal rats born of heavily infected mothers, but none of them was positive by in-situ hybridization. Five weeks after birth, cystic forms were detected in four neonatal rats. In the lobes of the lungs, no predilection site of P. carinii was recognized. Counts of cystic forms on smears and the reactivity of in-situ hybridization in the lungs of neonatal rats were significantly lower than in maternal rats. The present findings suggest that P. carinii is rarely transmitted through the placenta and proliferates less successfully in the lungs of neonatal rats than in mothers.
INTRODUCTION
Pneumocystis carinii is the most important opportunistic pathogen to inhabit immunocompromised hosts, where it is known to develop by active proliferation during latent infection (Sheldon, 1959). This theory is supported by the fact that most of humans are exposed to P. carinii infection in their early life, 2 to 4 years after birth (Peglow et al., 1990; Hong, 1991). It has therefore been speculated that certain stages of P. carinii dwell unnoticed in the lungs of normal humans. During immune derangement, these organisms proliferate, occupy the alveolar cavities, and finally induce clinical pneumonia. This hypothesis was supported by demonstration of latent infection in rat experimental models (Bartlett et al., 1987).
Case reports describing the existence of P. carinii pneumonia in a family (Yates et al., 1975) and in hospitalized immunosuppressed patients (Chave et al., 1991) have suggested, however, the importance of new transverse transmission rather than activation of organisms in latent infection. According to a study of P. carinii based on a case of recurrent pneumocystosis, each recurrent isolate showed genetic variation, and this was regarded as an evidence of new infection (Keely et al., 1995). In addition, detection of P. carinii using toluidine blue-O stain, immunohistochemical techniques or polymerase chain reaction showed that latent infection was rather rare in humans (Ognibene et al., 1988; Peters et al., 1992). These findings, therefore, strongly support the suggestion that P. carinii pneumonia is usually induced by transverse transmission and latent infection does not last as long as previously thought.
Many cases of ectopic pneumocystosis have involved almost all organs (Raviglione, 1990). Extrapulmonary spread of these organisms might occur in a limited number of AIDS-related situations (Radin et al., 1990), and in addition a few case reports have suggested the possibility of transplacental or intrauterine vertical transmission of P. carinii (Pavlica, 1962; Bazaz et al., 1970). These are, however, difficult to believe; to date, no experimental data have proved the existence of vertical transmission. The present study investigated experimental induction of vertical transmission of P. carinii in neonatal rats born of heavily infected mothers.
MATERIALS AND METHODS
Maternal rats
Adult female Wistar rats were purchased from the laboratory animal center of Seoul National University, Seoul, Korea, in 1996. They were reared on regular commercial diet and tap water, and assigned to groups I, II or III according to immunosuppression and animal room (Table 1). Total of 30 female rats in group I were immunosuppressed by subcutaneous injection of 4 mg methyl-prednisolone (Depomedrol®, Upjohn Korea Co., Seoul), once a week during the experi-mental period. Group II consisted of 30 normal female rats which were kept in the same animal room as group I rats, and 30 other rats (group III) were kept in another animal room. Table 1 shows the numbers of maternal or neonatal rats included in the results.
Mating
One normal adult male rat was supplied to five female rats for mating; those in group I were mated after 3 weeks of steroid injection. Pregnant female rats were separated and each was kept single until the end of experiment. A total of 23 immunosuppressed pregnant rats in group I died before delivery, and they were excluded from the experiment.
Neonatal rats
One half of the neonates from one maternal rat were immunosuppressed by injection of 0.5 mg methyl-prednisolone, once a week after delivery, while the other half received no special treatment and were kept in the same cage with their mother and immunosuppressed siblings. Many neonatal rats died before one week of delivery, and they too were excluded.
Quantification of P. carinii cystic forms
All maternal and neonatal rats were sacrificed between weeks 1 and 5 of experiment by ether anesthesia and neck dislocation, and their lungs were removed. Three impression smears per rat were taken from the upper, middle, and lower lobe of the right lung. They were stained by Diff-Quik solution (Fisher Scientific Co., USA), and cystic forms of P. carinii with intracystic bodies were counted individually in 20 fields of immersion oil lens magnification for maternal rats, while for neonatal rats, whole fields were screened.
In-situ hybridization
The procedures and materials for in-situ hybridization were as described by Park et al. (1991) and Montone (1994), and the Microprobe System (Fisher Scientific Co.) was used. A 22-mer oligonucleotide probe (5S ribosomal DNA: 5' TCT CTG AGG TAT GGC GGC CGT AAC T 3') specific to P. carinii (Kim et al., 1996) was synthesized and labelled with biotin on its 3' terminal (Korea Biotec Co., Korea). The left lungs were formalin-fixed and paraffin-embedded, and the lung tissue was cut into 5-µm-thick sections prior to mounting on Pro-On glass slides (Fisher Scientific Co.). This was followed by deparaffinization with a dewaxing agent (Histochoice, Amresco, USA). The sections were washed with absolute ethanol, rehydrated with universal buffer (Research Genetics, USA), and digested with 2.0 mg/ml pepsin (Research Genetics) for 3 min at 100℃. The probe was diluted to 20 pmol/ml in Brigati probe diluent (Research Genetics), and hybridized to the tissues for 30 min at 65℃. The slides were washed twice in 2X SSC at room temperature. The biotinylated probe was detected by incubating for 10 min at 45℃ with avidin-alkaline phosphatase (Biomeda Co., USA); the chromogen used was Fast Red TR salt (Amresco). Tissues were counterstained with hematoxylin and coverslipped by universal mount. The reactivity of each slide was graded according to the stained area; reactivity in minimal focus was graded as +1; single layer reactivity in the alveoli, less than one-third of the lung section, was graded as +2; several layers of reactivity in the alveoli, more than one-third and less than two-thirds, was graded as +3; and alveolus-filling reactivity in most of the lung section was graded as +4.
Statistical analysis
The counts of cystic forms in the lungs of maternal rats and neonates between groups were examined by the Wilcoxon rank sum test, and the ANOVA test was used to analyse the counts by the lung lobes.
RESULTS
P. carinii infection in maternal rats
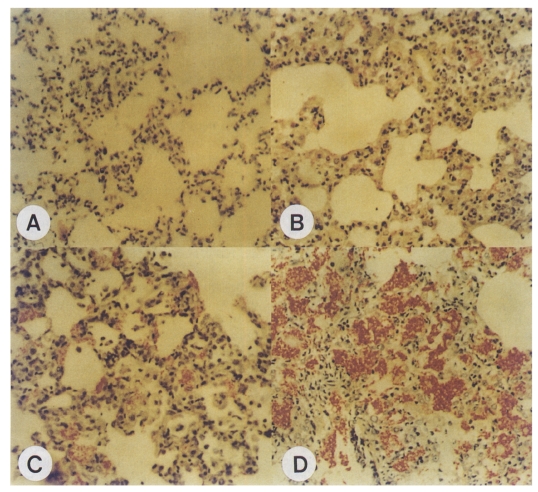
Cystic forms of P. carinii were found in all impression smears of group I maternal rats (Fig. 1A, Table 2), and all of them also reacted positively during in-situ hybridization (Table 3). There was, however, no evidence of P. carinii infection in maternal rats of groups II and III (Tables 2, 3).
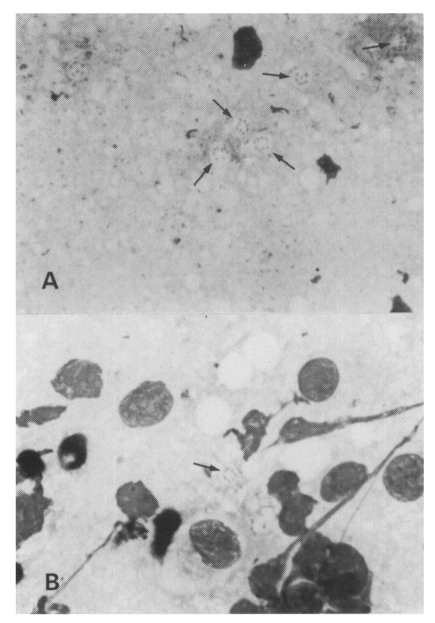
A. Impression smear of the right upper lobe of the lung of a maternal rat; Group I, Diff-Quik stained. Many cystic forms with intracystic bodies (arrows) are observed, original magnification × 1,500. B. Impression smear of the right upper lobe of the lung of a neonatal rat; Group I, Diff-Quik stained. A cystic form (arrow) is observed, original magnification × 1,500.
Recovery of Pneumocystis carinii cystic forms, as seen on Diff-Quik stained impression smears of the lungs by weeks after birth
P. carinii infection in neonatal rats
Cystic forms of P. carinii were found 1 and 5 weeks after birth only in neonates born to group I maternal rats (Fig. 1B, Table 2), while no neonates born to groups II and III showed cystic forms on their impression smears (Table 2). One week after birth, three of six immunosuppressed neonates showed the cystic forms of P. carinii on impression smears, but in-situ hybridization showed that all six were negative (Table 3). Five weeks after birth, all four neonates from group I maternal rats showed P. carinii cystic forms on impression smears regardless of immunosuppression (Table 2), in-situ hybridization showed positive reactivity in three of the four (Table 3). One normal two-week-old neonate from a maternal group II rat showed positive reactivity by in-situ hybridization; it had recieved no steroid injection (Table 3).
Quantification of P. carinii cystic forms
All immunosuppressed maternal rats in group I were infected by P. carinii, and their impression smears included cystic forms from 1 to 436 (average, 152) in 60 fields of immersion oil lens magnification (Table 4). In the seven positive group I neonates, 1-5 (average, 3) cystic forms were counted in the whole field of smears. The Wilcoxon rank sum test showed statistical significance of the cystic form counts between mothers and neonates (p=0.032), but the ANOVA test showed no statistical significance of the counts between the lung lobes (p=0.989).
Relationship between the number of cystic forms seen on impression smears and reactivity by in-situ hybridization
No impression smears of immunocompetent maternal rats (groups II and III) and their neonates revealed cystic forms; in-situ hybridization revealed minimal reactivity (+1) in only one 3-week-old group II neonate (Table 3). In-situ hybridization of group I immunosuppressed maternal rats indicated positive reactivity proportional to counts of cystic forms (Table 4). In rats with one or two cystic forms there was no reactivity (-); in those with 5 to 6 forms there was minimal reactivity (+1), and those with 72 or 86 counts forms showed moderate reactivity (+2). In those with 146 or 319 forms, reactivity was severe (+3), and in those with 436, very severe (+4).
DISCUSSION
The present findings strongly suggest that vertical transmission of P. carinii from an infected mother to an intrauterine fetus is rare. A few reports have described a number of plausible cases of intrauterine infection (Pavlica, 1962; Bazaz et al., 1970; Pifer et al., 1984) and extrapulmonary spread of P. carinii in most viscera has been verified (Raviglione, 1990), but no experiments have yet proved vertical transmission. If this were common, most neonatal rats born of heavily infected group I mothers would have been infected. A contradicting report stating that a neonate delivered from a heavily infected mother with P. carinii was not infected with this organism has also been published (Gates and Barker, 1993). Furthermore, according to an epidemiological study of vertical HIV infection in infants, all P. carinii infected cases were detected three months after birth (Berger et al., 1995), suggesting less possibility of vertical infection by P. carinii.
The three 1-week old immunosuppressed neonatal rats from group I maternal rats showed a few cystic forms of P. carinii on impression smears but not by in-situ hybridization. The result could be interpreted as indicating the presence of a few cystic forms of P. carinii but no active proliferation in the lungs. The transmission of cystic forms to these three neonates might have been vertical (before birth) or horizontal (after birth). If they had been vertically infected, the organisms could have proliferated in the lungs for about two weeks and in-situ hybridization would have been at least minimally positive. The present finding thus indicates that the cystic forms found in the lungs of neonates were horizontally transmitted after birth through the air. The forms are evidence of initial exposure of neonatal rats to P. carinii immediately after birth. In addition, the present authors have an experience of detecting no cystic forms in fetal lungs from infected mothers (data not shown).
Furthermore, 2- or 3-week old neonates from group I infected maternal rats were free from P. carinii regardless of immunosuppression. If cystic forms actively proliferated in the lungs of neonates, more organisms would be recovered than in 1-week old neonates. The present finding therefore suggests that the cystic forms found in the lungs of 1-week old neonates hardly proliferated.
In the lungs of four 5-week old neonates from group I severely infected maternal rats, cystic forms were found on impression smears, and in three of their in-situ hybridization was also positive. In individual rats, cystic forms numbered between two and six, and in-situ hybridization reactivity was minimally positive (+1); this finding indicates the initial proliferation of organisms in the lungs. In addition, the recovery and intensity of P. carinii from the lungs of 5-week old group I neonates showed no difference between untreated and immunosuppressed individuals, and it may thus be induced that untreated neonates were immunosuppressed because their mothers were severely immunosuppressed during pregnancy and lactation.
The present findings for group I neonatal rats confirm that cystic forms of P. carinii were transmitted through the air from mothers to neonates within one week after birth and that the organisms began to proliferate five weeks after birth. There was no evidence that P. carinii proliferated in the lungs of immunosuppressed neonates until three weeks after birth, a finding quite different from that of adult Wistar rats, in which infection is detectable within three weeks of immunosuppression (Hong et al., 1994). Additionally, the counts of cystic forms were very low compared to those of adult rats five weeks after immunosuppression.
There are several plausible ways of explaining this gap of timing and intensity for P. carinii infection in neonatal rats. The first is that the composition of surfactant in a neonate's lung does not favor proliferation of P. carinii. Ohashi et al. (1994) measured the amount of surfactant protein A (SP-A) and saturated phosphatidylcholine (Sat PC) in the alveolar wash of rats of various ages. They found that the total amount of these substances increased with age but that the concentration of SP-A and Sat PC by surface area was highest on day 1 and decreased thereafter. The ratio of SP-A to Sat PC ratio, which is known to be related to proliferation of P. carinii, was higher between days 21 and 50 than at other ages. In rabbits, no organism was detected for 20 days after birth, while the ratio remained high, and P. carinii proliferated as the ratio decreased (Aliouat et al., 1998). This finding from the rabbits correlates closely with the present findings for rats, thought to be due to surfactant composition. The second explanation is that the effect of prednisolone on the composition of surfactant possibly differs between neonates and adults, though no concrete data are available. The third possibility is that passively transmitted maternal immunoglobulins or cytokines protect neonatal rats from P. carinii infection during the first three postnatal weeks, while passive immunity from maternal rats is active. More studies are necessary to explain the mechanism of less proliferation of P. carinii in neonates than in adult rats.
In conclusion, vertical transmission of P. carinii is very rare. Neonates born of an infected mother become infected one week after birth, but no organisms proliferate during the first three weeks of life. The lungs of neonates may be unsuitable for proliferation of these organisms.