Growth and development of Gymnophalloides seoi in immunocompetent and immunosuppressed C3H/HeN mice
Article information
Abstract
The growth and development of Gymnophalloides seoi were studied in C3H/HeN mice and effects of immunosuppression of the host on the worm development were observed. Two hundred metacercariae of G. seoi were orally administered to each mouse, and worms were recovered on days 1, 3, 5, 7, 14 and 21 post-infection (PI). The worm recovery rate was significantly higher in immunosuppressed (ImSP) mice than in immunocompetent (ImCT) mice except on days 1 and 3 PI. The worms attained sexual maturity by day 3 PI with eggs in the uterus, and worm dimensions and the number of uterine eggs continuously increased until day 14 PI in ImSP mice. Worms recovered from ImSP mice were significantly larger in size than those from ImCT mice on days 1 and 3 PI, and the number of uterine eggs was significantly larger in ImSP mice on days 5 and 7 PI. Genital organs such as the ovary, testes, and vitellaria, that were already developed in the metacercarial stage, grew a little in size until day 14 PI. The results show that the C3H/HeN mouse is, though not excellent, a suitable laboratory host for G. seoi.
INTRODUCTION
Gymnophalloides seoi is a new intestinal fluke of humans contracted by eating raw oysters in Korea (Lee et al., 1993, 1994, 1995). Some of the infected people have suffered from acute pancreatitis and gastrointestinal troubles (Lee et al., 1993, 1994). In other cases, pathogenicity of G. seoi to humans is still unknown. To study the pathogenicity and host-parasite relationships, and to obtain living adult flukes, suitable animal models are greatly needed.
Migrating birds are generally known to be natural final hosts of gymnophallid trematodes (Ching, 1995). But birds are not a useful experimental animal and requirements for a suitable laboratory host have been increasing. For being a suitable host for a parasitic infection, the animal should be genetically susceptible to the infection (Chai et al., 1984), growth and development of worms should be excellent, and longevity of worms be sufficiently long.
In this connection, a study searching for a suitable animal host for G. seoi was performed, and the C3H/HeN mouse that showed a fairly high susceptibility to an experimental infection was proposed as a suitable laboratory host (Lee et al., 1997). However, the detailed growth and development of worms have not been studied in this mouse strain. Therefore, the present study was performed to observe the growth and development of G. seoi in C3H/HeN mice and to determine whether the development of worms is enhanced by immunosuppression of the host.
MATERIALS AND METHODS
Collection of metacercariae
Naturally infected oysters, Crassostrea gigas, were collected from Shinan-gun, the known endemic area of gymnophalloidiasis (Lee et al., 1994). The oyster shell was removed, and the animal part was slightly digested in artificial digestive juice (0.5% pepsin 1:10,000 in 0.6% HCl solution; Sigma, St. Louis, USA) at 37℃ for 3 min. The digested material, which contained freed metacercariae, was washed several times with normal physiological saline. After repeated sedimentation and washing the metacercariae were collected under a stereomicroscope.
Animal, experimental infection, and worm recovery
C3H/HeN mice, 4-5 weeks old and weighing 20-25 g, were used as the experimental host. Two hundred metacercariae were orally administered to each mouse, and the worms were recovered at days 1, 3, 5, 7, 14 and 21 post-infection (PI). To observe the effects of immunosuppression on the worm development in C3H/HeN mice, dexamethasone (KGMP, Korea) at a daily dose of 2 mg/kg was injected intramuscularly every day from day 7 before-infection to the day of sacrifice. Untreated and dexamethasone-treated mice were designated as immunocompetent (ImCT) and immunosuppressed (ImSP) mice, respectively.
Observation of worm growth and development
The worms recovered were fixed in 60℃ 10% formalin without applying pressure, and stained with Semichon's acetocarmine (BDH Chem., England). Dimensions of worms were measured, the number of uterine eggs was counted, and the development of genital organs was observed using a light microscope.
Statistical analysis
Values were expressed as the mean ± standard deviation (SD) of data from more than 10 worms. Statistical significance between groups was tested by the Students' t-test.
RESULTS
Worm recovery rates
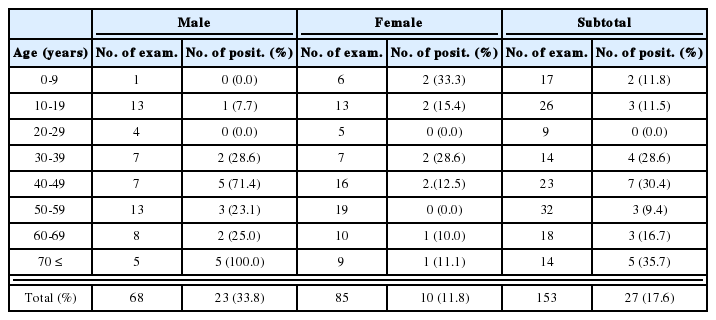
Worm recovery rates of G. seoi were significantly higher in ImSP mice than in ImCT mice through days 5-21 PI (P<0.05) (Table 1). On days 1 and 3 PI, however, the rates in ImSP mice were not higher than in ImCT mice (Table 1). On days 14 and 21 PI, no worms were recovered from ImCT mice, whereas a few worms were steadily recovered from ImSP mice (Table 1).
Growth of worm length and width
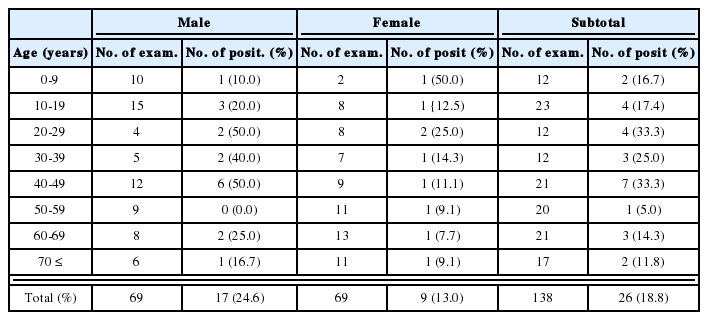
Dimensions of worms recovered from ImSP mice were significantly larger than those recovered from ImCT mice on days 1 and 3 PI (P<0.05) (Table 2). Also, in ImSP mice, worm dimensions continuously increased during day 1-14 PI and slightly decreased on day 21 PI (Table 2). In ImCT mice, however, the worm attained its maximum growth on day 5 PI, after which they showed no more recognizable growth in size (Table 2).
Growth and development of body organs
The growth in size of non-genital organs (i.e., oral and ventral suckers) was very slow, negligible, and not significantly different between worms from ImCT and ImSP mice (P>0.05) (Fig. 2). However, genital organs such as the ovary, testes, and vitellaria were already well developed in the metacercarial stage and further developed quickly to their functional maturity within days 2-3 PI, as shown by the appearance of uterine eggs on day 3 PI (Fig. 1). The seminal vesicle and seminal receptacle were not yet recognizable in 1-day worms, but seen to have developed by day 3 PI. The size development of sexual organs by infection days was very slow and not significantly different between worms recovered from ImCT and ImSP mice (P<0.05) (Table 3).

Growth curves of oral and ventral suckers of Gymnophalloides seoi recovered from ImCT or ImSP C3H/HeN mice. Worms were not recovered from ImCT mice at days 14 and 21 PI. No significant difference was noted between worms from two groups of mice (P>0.05).

Growing patterns of Gymnophalloides seoi in C3H/HeN mice. A. excysted metacercaria, B. 1-day old worm, C. 3-day old worm, D. 5-day old worm, E. 7-day old worm, F. 14-day old worm.
Number of uterine eggs
Eggs were not seen in uteri of 1-day old G. seoi recovered from ImCT or ImSP mice (Fig. 1). But they began to appear in 3-day old worms in both groups. In ImCT mice, the number of eggs significantly increased in accordance with the aging of worms (P<0.05) (Fig. 3). Worms recovered from ImSP mice had more eggs in the uterus than ImCT mice on days 3, 5 and 7 PI, although the difference was statistically insignificant in 3-day old worms (P>0.05). In ImSP mice, the number of eggs significantly increased according to the aging of worms (P<0.05) (Fig. 3).
DISCUSSION
In this study, to reduce technical bias in measuring dimensions of worms which may occur during worm fixation, hot (60℃) 10% neutral buffered formalin was used and no cover slip pressure was applied during the fixation. By this method worms were quite well fixed, and most of them were not severely contracted but smoothly relaxed. A disadvantage of this method was unclear morphology of small internal organs and structures.
The present study revealed that G. seoi grew successfully in C3H/HeN mice containing many eggs in the uterus. Therefore, C3H/HeN mice are evaluated as a suitable laboratory host. Moreover, immunosuppression of these mice brought about a higher worm recovery rate, longer survival, and better growth and development of worms. The higher worm recovery rate from ImSP C3H/HeN mice was consistent with our previous report (Lee et al., 1997). But the better growth and development of worms in ImSP mice than in ImCT mice were in disagreement with our previous study (Lee et al., 1997). A further detailed study would be helpful to explain this discrepancy. The lower worm recovery rate on day 1 PI from ImSP mice than ImCT mice was also difficult to explain. Technical errors to recover all of the 1-day old worms can not be ruled out completely.
However, since the size of worms recovered in this study was much smaller than those recovered from humans (Lee et al., 1993) and the worm recovery rate even from ImSP mice was not sufficiently high, it is difficult to regard C3H/HeN mice as an excellent laboratory host for G. seoi infection. The biggest and most ovigerous worms were recovered from ImSP mice on day 14 PI, and the size of worms as well as the number of uterine eggs decreased in worms of 21 days old. This result represents that G. seoi could not survive for a sufficient period in C3H/HeN mice even though the host is immunosuppressed, and supports the suggestion that this mouse strain is not an excellent host animal.
It is interesting to note that G. seoi attained sexual maturity very quickly, by day 3 PI, in C3H/HeN mice. A similar finding was reported using in vitro culture of G. seoi (Kook et al., 1997). It seems greatly due to the early development of primordia of the ovary, testes, and vitellaria in the metacercarial stage (Lee et al., 1995). Sperms and ova seem to be produced directly after infection to the final host, and eggs be produced subsequently.
The duration for trematodes to be ovigerous has been reported variable. In case of Neodiplostomum seoulense, for example, primordia of reproductive organs were not recognizable in the metacercarial stage, and the ovary appeared only 3 days after experiemental infection to rats, and they became ovigerous on day 5 PI (Hong, 1982). On the other hand, in case of Heterophyopsis continua, the ovary and testes were already present in the metacercarial stage, and the presence of uterine eggs required only 3 days (Hong et al., 1990).
Although it was successful to culture G. seoi metacercariae in vitro, with mature reproductive organs and eggs in cultured worms (Kook et al., 1997), the extent of growth and development of worms was not comparable with those recovered from C3H/HeN mice. This result indicates that in vitro conditions are less satisfactory for the development of worms, compared with in vivo conditions.
In conclusion, the C3H/HeN mouse is, though not excellent, a suitable experimental host for G. seoi. It was worth to note that the worm growth, development, and survival is enhanced by immunosuppression of the host.
Notes
This study was supported in part by the Basic Medical Research Fund, Ministry of Education, Republic of Korea (1994).