Karyotypes on three species of Chinese mesogastropod snails, Semisulcospira libertina, S. dolichostoma and Viviparus rivularis
Article information
Abstract
Three species of the families Viviparidae and Pleuroceridae, the first intermediate host of paragonimiasis, metagonimiasis and echinostomiasis were studied cytologically. The observed diploid chromosome number was as follows: Semisulcospira libertina 36, S. dolichostoma 34, and Viviparus rivularis 64. The mitotic chromosome complement of S. libertina has nine metacentric pairs and nine submetacentric pairs, and S. dolichostoma has three metacentric pairs and 14 submetacentric pairs of chromosomes. Viviparus rivularis showed two metacentric pairs and 30 submetacentric pairs of chromosomes.
INTRODUCTION
Taxonomy of viviparid and pleurocerid snails which serve as intermediate hosts for several kinds of trematodes has been in a chaotic state for many years (Burch, 1975). The systematics of the lower taxonomic categories still remains an enigma, both from the practical viewpoint of identifying specimens and in understanding the mechanisms of speciation and evolution (Burch, 1968). In mollusca, the literatures on karyotype analysis are not abundant due to difficulties of obtaining mitotic fields with enough quality to carry out chromosome studies. "Melaniid" (=Pleurocerid) snails are among the most common and widely dis-tributed groups of freshwater mollusks in the world, however studies concerning their chromosomes are rare. Until now, the chromosomes of six species have been described among the poorly understood genera Semisulcospira and Viviparus occurring in China.
The greatest variation in chromosome number of any mesogastropod family occurs in the pleuroceridae. Haploid chromosome number in 22 species and one subspecies of Semisulcospira ranges from n=7 to n=20 (Patterson, 1969; Dillon, 1991; Nakano et al., 1994). The chromosome number of the family Viviparidae ranges from n=7 to n=14 by the literature survey (Ramamoorthy, 1958; Inaba, 1965; Park et al., 1997). In the subfamily Viviparinae, the haploid numbers of eight species range from n=7 to n=13 (Patterson, 1969; Zhou et al., 1988).
The purpose of this study was to detect the chromosome numbers and to identify the karyotypes of two pleurocerid and one viviparid Chinese snails, which serve as molluscan intermediate hosts for paragonimiasis, metagonimiasis and echinostomiasis.
MATERIALS AND METHODS
The snails in this study were collected from Houwei and Suncun Villages, Jinde County, Anhui Province, China, in October 1997. Chromosome preparations were made from gonadal tissues by the air-drying method (Park, 1994). The materials examined were gonads in active stages of gametogenesis from six specimens of Semisulcospira libertina, five specimens of S. dolichostoma and three specimens of Viviparus rivularis. To observe morphological features of the chromosomes, karyotypes, relative and total lengths of the mitotic metaphase chromosomes were measured. Nomenclature of morphological types of chromosome was adopted from Levan et al. (1964).
RESULTS
Pleuroceridae
Semisulcospira libertina
Chromosomes were observed in three males and three females. The mitotic metaphase chromosomes were observed in both sexes. The diploid chromosome number was 36 (Fig. 1). The karyotypes were arranged by shape and size (Fig. 5), and Table 1 shows the results of chromosome measurement from this species. Chromosomes in males and females ranged from 2.2 to 4.2 µm in length. Mean total length of the diploid complements were 49.5±2.72 µm. Karyotype consists of nine pairs of metacentrics and nine pairs of submetacentric chromosomes (Fig. 5).
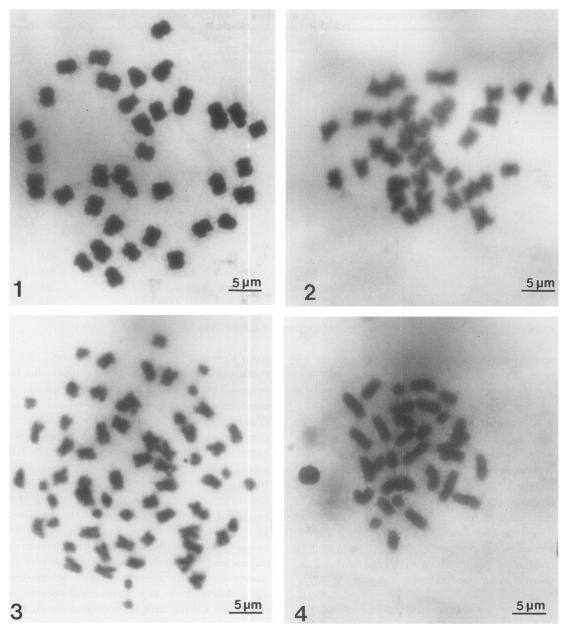
Meiotic and mitotic chromosomes of three Chinese snail species.
Fig. 1. Diploid chromosomes in metaphase I of Semisulcospira libertina. Fig. 2. Diploid chromosomes in metaphase I of Semisulcospira dolichostoma. Fig. 3. Diploid chromosomes in metaphase I of Viviparus rivularis. Fig. 4. Haploid chromosomes of V. rivularis.
Semisulcospira dolichostoma
Thirty-four chromosomes were observed at spermatogonial metaphase in 24 mitotic cells studied. The mean total chromosome length, based on measurements from three sets of karyotyped cells, was 50.3±2.29 µm. The length of chromosomes ranged from 2.1 to 4.8 µm (Table 2). Fig. 6 shows the karyotype constructed from the chromosomes shown in Fig. 2, which was one of the most elongated complements. This species has 34 chromosomes: three pairs of metacentrics and 14 pairs of submetacentrics.
Viviparidae
Viviparus rivularis
From three male specimens, spermatogonial mitotic divisions were observed in 18 cells. In these cells, 64 chromosomes (2n) were also counted (Fig. 3). Meiotic chromosomes in male were observed (Fig. 4). The lengths of the chromosomes in male ranged from 1.6 to 4.6 µm. Mean total length of the diploid complements in male were 96.4±7.86 µm (Table 3). Karyotypes consist of two pairs of metacentrics and 30 pairs of submetacentric chromosomes (Fig. 7).
DISCUSSION
One of the most commonly encountered genera of freshwater snails in the Far East Asia is Semisulcospira. More than 18 species and subspecies of this genus have been described from China (Yen, 1939). Identification of these species is difficult due to the great amount of variation in shell shape, structure and coloration. In the Pleuroceridae, the family to which Semisulcospira is presently referred about chromosomes, 12 species have been studied from Japan and eight species from Korea (Patterson, 1967; Burch, 1968; Park, 1994). Semisulcospira libertina (=bensoni) is regarded as the most important intermediate host of Paragonimus westermani (Yokogawa et al., 1960a, 1960b). Several nominal subspecies of S. libertina have also been implicated as intermediate hosts for paragonimiasis and metagonimiasis in humans and other mammals in the Far East. However, a little is known concerning the biological relationships of the nominal species of Semisulcospira over its wide range of geographic distribution (China, Formosa, Japan and Korea), and the relationship of S. libertina to other melaniid snails. Semisulcospira dolichostoma is a pleuracerid species in mainland China, and no information on the susceptibility of human-infecting trematodes is available. Although some viviparid snails such as Cipangopaludina spp. act as the second intermediate host of echinostome trematods, the parasitological role of V. rivularis has not been reported yet. Therefore, further studies on the susceptibility tests of these snails to trematodes are needed. Patterson (1967) found 18 bivalents in each of the specimens of S. libertina that were studied from four localities in Japan. Inaba and Tanaka (1953) reported S. libertina to have eight pairs of chromosomes (n=8, 2n=16). In this study, however, all specimens of this species had 18 bivalents. Burch (1968) also found S. libertina to have a haploid chromo-some number of n=18. We therefore mention that Inaba and Tanaka's report (1953) of only eight pairs of chromosomes for this species is in error. Burch (1968) extended our cytological knowledge of Semisulcospira by studying eight additional species in which the haploid chromosome numbers ranged from n=8 to n=20. With such a wide variation in chromosome numbers, the pleuroceridae has been proven to be a group of considerable cytogenetic interest. Park (1994) has reported on the chromosomes of eight species of pleuroceridae in Korea. The six species of Semisulcospira in his study had chromosome number of n=18 and 2n=36. It is obvious that chromosome number is more conserved in species of Semisulcospira in Korea than those in Japan or China. Park (1994) reported that the karyotyping is especially helpful for analyzing Semisulcospira in Korea, since all species studied so far from Korea have the same chromosome number (n=18, 2n=36). Semisulcospira forticosta and S. tegulata can be distinguished by their karyotypes.
In the Viviparidae, the chromosome number of all species studied so far is n=7 to n=14 (Patterson, 1969; Zhou et al., 1988; Park et al., 1997). In the most primitive viviparid subfamily, Bellamyinae, the haploid chromosome numbers of 11 species studied range from 8 to 11 (Ramamoorthy, 1958; Inaba, 1965; Zhou et al., 1988). In the subfamily Viviparinae, the haploid number of eight species ranges from n=7 to n=13 (Rainer, 1963). Recently, Park et al. (1997) reported the chromosome numbers in two species of echinostome vector snails of the family Viviparidae in Korea. In the present study, however, the chromosome number of V. rivularis is different from those of the former reports.
According to the above comparison, it is suggested that S. libertina is more conserved than S. dolichostoma or V. rivularis. DNA sequence analysis of specific genes could be valuable for further classification of the relationship of vector snails in different localities.
Notes
This study was supported by a grant of the '97 Good Health Research and Development Project, Ministry of Health and Welfare, Republic of Korea.