Antibody reaction of human anti-Toxoplasma gondii positive and negative sera with Neospora caninum antigens
Article information
Abstract
Anti-Neospora caninum antibody was detected in anti-Toxoplasma gondii positive and negative human sera by ELISA, western blot and immunofluorescence assay (IFA). Twelve cases out of 172 (6.7%) Toxoplasma-positive sera cross-reacted with both T. gondii and N. caninum antigens, and one out of 110 Toxoplasma-negative sera reacted with N. caninum antigen by ELISA. By western blot, all 12 sera reacted with T. gondii antigens with various banding patterns but specifically at 30 kDa (SAG1) and 22 kDa (SAG2) bands. With N. caninum antigen, the number of reactive bands was reduced, however a 43 kDa band reacted in three cases in Toxoplasma-positive sera in addition to one in Toxoplasma-negative control sera. All sera of the Toxoplasma-positive group labeled surface membrane of T. gondii, but reacted differently with N. caninum. Fluorescence was detected in surface membrane, subcellular organelles, or both in N. caninum. And one case in the Toxoplasma-negative group also reacted with N. caninum strongly in subcellular organelles. This suggested that the antibody against N. caninum may be present in human sera although the positive rate was very low in this study. The possibility of human infection with N. caninum remains to be evaluated further.
INTRODUCTION
Neospora caninum is a protozoan (Apicomplexa) parasite that was first identified in 1988 during a retrospective study of dogs previously diagnosed with fatal toxoplasmosis (Dubey et al., 1988). This parasite causes serious neurologic and muscular diseases in dogs, cattle, sheep, goats and horses (Dubey and Lindsay, 1993). However, this coccidian has not been found in humans until now, but it has been reported that it can infect monkeys experimentally (Barr et al., 1994). Neospora caninum shares many features with, but is clearly distinct from Toxoplasma gondii. Before 1988, N. caninum was misdiagnosed as T. gondii because of the existence of morphologic and biologic similarities of tachyzoites between the two coccidia and their ubiquitous host ranges. In addition, there appears to be serologically cross-reactive due to shared antigens between N. caninum and T. gondii (Bjerkas et al., 1994).
However, they are differentiated genetically (Marsh et al., 1995) and evolve divergently (Guo and Johnson, 1995). More clearly, they are different from each other in antigenicity of major surface proteins; for example, SAG1 (p30) and SAG2 (p22) of T. gondii are absent in N. caninum (Brindley et al., 1993), whereas a 43 kDa (Nc-p43) protein, a major surface protein of N. caninum, is absent in T. gondii (Hemphill and Gottstein, 1996).
We tried to detect antibody against N. caninum in anti-Toxoplasma positive and negative human sera to differentiate antigenic cross reactivity and to seek unique reactions with N. caninum which could suggest a possibility of zoonotic infection of this parasite.
MATERIALS AND METHODS
Antigen preparation
RH tachyzoites of T. gondii were maintained by a peritoneal passage in mice and purified by centrifugation over 40% Percoll (Pharmacia, Sweden). Tachyzoites of N. caninum were maintained in Vero cell monolayer in DMEM supplemented with 10% FBS (Gibco, USA), and they were purely obtained from the supernatant of 3-4 days culture.
Sera
A total of 172 anti-Toxoplasma positive sera was composed of 75 indirect latex agglutination (ILA) positive cases (> 1:32) (Choi et al., 1989), 76 ILA and ELISA positive cases from Choi et al. (1992), 8 toxoplasmosis patients (Choi et al., 1997), and 13 ELISA positive cases screened. Anti-Toxoplasma negative sera were collected from 110 healthy students previously screened by ELISA.
Analysis of antigenic cross reaction
ELISA was performed according to the method of Choi et al. (1992). Briefly, T. gondii and N. caninum extracts, 1 µg/well each, were used as antigens. Sera diluted at 1:100 with PBS/0.05% Tween-20 and HRP-conjugated goat anti-human IgG antibody (Cappel, USA) diluted at 1:1,000 were used as the primary and secondary antibodies, respectively. Substrate solution was composed of 1 ml 1% ο-phenylene diamine, 50 µl of 30% H2O2 in 99 ml distilled water. After stopping the reaction with H2SO4, absorbances were read at 490 nm with an ELISA reader (Dynatech, USA).
Western blot was done by the method of Towbin et al. (1979). Nitrocellulose (NC) papers were incubated with sera with 1:500 dilution and then with 1:2,000 diluted HRP-conjugated goat anti-human IgG antibody. They were soaked in ECL solution (Amersham, USA) for 1 min and exposed to X-ray film (Fuji, Japan) for 5 to 10 sec.
For immunofluorescence assay (IFA), freshly prepared T. gondii and N. caninum tachyzoites suspended in PBS were cytospinned onto 18-mm coverslips. Tachyzoites were fixed with cold absolute methanol for 5 min. Sera were applied with 1:100 dilution in incubation solution (3% BSA in PBS) and coverslips were incubated with 1:500 diluted FITC-conjugated goat anti-human IgG antibody (Cappel). Fluorescence was observed under a fluorescence microscope (Axiophot, Zeiss Co.).
RESULTS
By ELISA, the cut-off value of anti-Neospora positive was determined as an absorbance of 0.24 (mean + 3SD) (Table 1). According to this cut-off value, 12 cases out of 172 (6.7%) Toxoplasma-positive sera showed cross reaction with both T. gondii and N. caninum antigens, and one case out of 110 in the Toxoplasma-negative sera reacted with N. caninum antigen. There were no significant correlations in absorbances between T. gondii and N. caninum as shown in Figure 1.
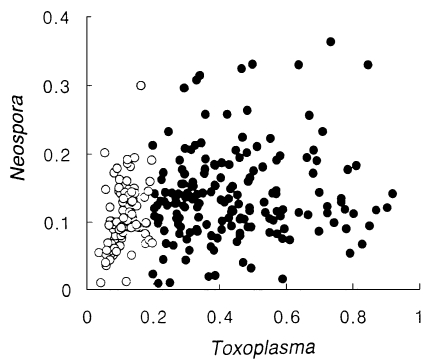
Distribution of absorbance spots of Toxoplasma vs. Neospora. Closed circles indicate the Toxoplasma-positive group and open circles indicate the Toxoplasma-negative group.
Cross-reacted sera were subjected to western blot with both T. gondii and N. caninum antigens. As shown in Figure 2, all 12 sera reacted with T. gondii antigens with various banding patterns, however specific reactions were observed at 30 kDa (SAG1) and 22 kDa (SAG2) bands (Fig. 2A). When the same sera were applied to N. caninum antigen, the number of reactive bands was reduced. But a 43 kDa band was observed in three sera in Toxoplasma-positive group (lanes 1, 7 and 10 in Fig. 2B) in addition to one in Toxoplasma-negative group.
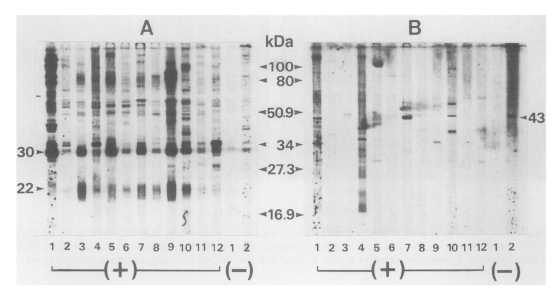
Western blot pattern of human sera with Toxoplasma gondii (A) and Neospora caninum (B) antigens. Sera were applied to the same lanes to both antigens, (+) means 12 cases of cross reactions from the Toxoplasma-positive group by ELISA and (-) means 2 cases from the Toxoplasma-negative group.
These sera were tested further by IFA (Fig. 3). All sera of the Toxoplasma-positive group labeled surface membrane (subcellular organelles also in some cases) of T. gondii, but reacted differently with N. caninum. In the case of lane 1 in Figure 2, the surfaces of both T. gondii and N. caninum were fluorescent (Fig. 3B). With the serum of lane 4, fluorescence of N. caninum was much brighter than that of T. gondii (Fig. 3C). Especially with the serum of lane 7, surface of N. caninum was labeled distinctly (Fig. 3D). The serum of lane 10 reacted with subcellular organelles of N. caninum (Fig. 3E). And the serum of Toxoplasma-negative lane 2 also reacted with N. caninum strongly in subcellular organelles (Fig. 3F). Sera of the other lanes reacted with T. gondii, but not with N. caninum (data not shown).
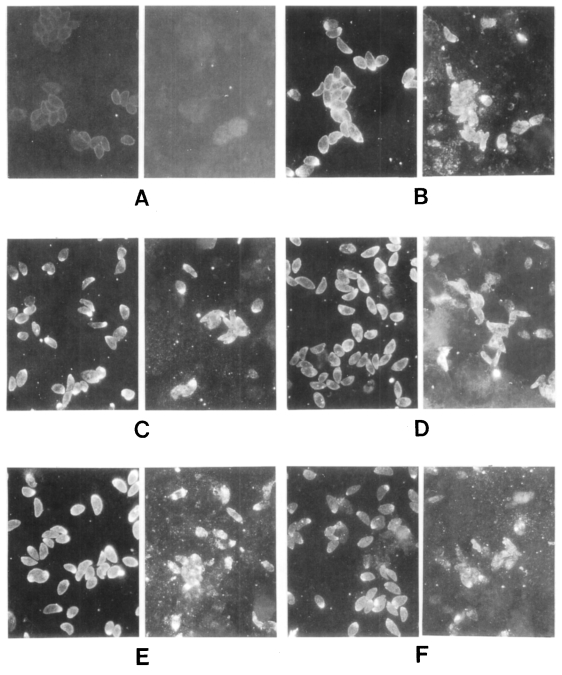
Immunofluorescence photomicrographs of Toxoplasma gondii (left) and Neospora caninum (right). A, cotrol with serum of lane 1 of the Toxoplasma-negative group; B, serum of lane 1; C, lane 4; D, lane 7; E, lane 10 of the Toxoplasma-positive group; F, lane 2 of the Toxoplasma-negative group, respectively.
DISCUSSION
Twelve out of 172 (6.7%) Toxoplasma-positive sera cross-reacted with both T. gondii and N. caninum antigens, and one out of 110 Toxoplasma-negative sera reacted with the N. caninum antigen by ELISA. By western blot, all of 12 sera showed various banding patterns, however they reacted specifically with 30 kDa (SAG1), and 22 kDa (SAG2) bands of T. gondii. With the N. caninum antigen, the number of reactive bands was reduced, but three sera in Toxoplasma-positive group and one in negative group reacted with a 43 kDa protein. The surface membrane of T. gondii was labeled with all Toxoplasma-positive sera. In case of N. caninum, fluorescence was detected in the surface membrane, subcellular organelles, or both. Interestingly, one Toxo-plasma-negative serum strongly reacted with the subcellular organelles of N. caninum.
We focused on the binding of 43 kDa protein in western blot using N. caninum antigen, which might be the most antigenic during infection because the Nc-p43 was identified as a major surface protein of N. caninum (Hemphill, 1996; Hemphill and Gottstein, 1996). In western blot of N. caninum, approximate molecular weights of 29, 30, 37, 51/52 kDa proteins were detected in addition to 43 kDa protein. These proteins were already characterized to be immunogenic, but they were not located in the surface membrane but in the rhoptries, dense granules, micronemes, tubular network, and membrane of the parasitophorous vacuole (Barta and Dubey, 1992; Bjerkas et al., 1994). Whereas proteins of subcellular organelles or specific DNA sequences of N. caninum have been identified one by one (Cole et al., 1994; Lally et al., 1996 & 1997), organelles and their functions are seemingly conserved evolutionarily within the phylum Apicomplexa. So there are proteins which cross-react with subcellular organelles of both species as demonstrated in IFA. Bjoerkman et al. (1994) characterized antigens that are currently used in an ELISA for diagnosis of N. caninum infections. This group of antigens comprised four proteins of 52-61 kDa, and three proteins of 31-36 kDa, the location of which are suggested to be associated with membrane. Moreover, without Nc-p43 binding in western blot, the surface membrane was detected to be fluorescent. Further study is needed to clarify the surface proteins of N. caninum including Nc-p43.
As repeatedly proven in antibody surveys for toxoplasmosis, the positive rates were not high in Korea (Choi et al., 1992) compared with those reported in European and American countries (Dubey and Beattie, 1988), which may be related with the differences in the food habit and/or the pet-loving behavior. Recently, dogs were identified as the definitive host of N. caninum (McAllister et al., 1998) and Kim et al. (1998) isolated N. caninum from a calf in Korea. Thus human may have a chance to be exposed to N. caninum antigen as an infective form or an antigenic form merely. With this study, although specific binding to the surface membrane was not differentiated between T. gondii and N. caninum, the presence of antibody against N. caninum in human sera could not be overlooked.
Notes
This study was supported by the Songeui Research Grant of Catholic Medical Center, 1998.
